
(a)
Interpretation:
The systematic name for the following compound should be determined.
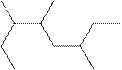
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(b)
Interpretation:
The systematic name for the following compound should be determined.
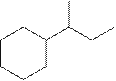
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which a series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and whose atoms don't form a ring is known as acyclic compounds. The general molecular formula of a cyclic alkane is
Rules of naming cycloalkanes are:
- First, determine the cycloalkane present in the structure which is considered as a parent chain (maximum number of carbon atoms). If the acyclic alkane chain has more carbon atoms, then the alkyl chain is considered a parent chain.
- For a cyclic system, the number of carbon atoms must be identified as present in different paths connected with two bridgeheads.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group or cycloalkyl group and denote its position on the parent chain with the number
- The alkyl groups or cycloalkyl groups are written in alphabetical order.
(c)
Interpretation:
The systematic name for the following compound should be determined.

Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(d)
Interpretation:
The systematic name for the following compound should be determined.
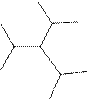
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(e)
Interpretation:
The systematic name for the following compound should be determined.
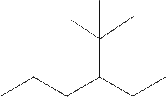
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(f)
Interpretation:
The systematic name for the following compound should be determined.
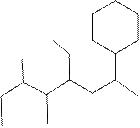
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(g)
Interpretation:
The systematic name for the following compound should be determined.
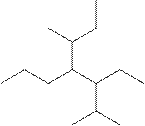
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(h)
Interpretation:
The systematic name for the following compound should be determined.
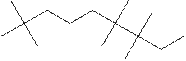
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(i)
Interpretation:
The systematic name for the following compound should be determined.

Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(j)
Interpretation:
The systematic name for the following compound should be determined.
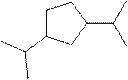
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which a series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and whose atoms don't form a ring is known as acyclic compounds. The general molecular formula of a cyclic alkane is
Rules of naming cycloalkanes are:
- First, determine the cycloalkane present in the structure which is considered as a parent chain (maximum number of carbon atoms). If the acyclic alkane chain has more carbon atoms, then the alkyl chain is considered a parent chain.
- For a cyclic system, the number of carbon atoms must be identified as present in different paths connected with two bridgeheads.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group or cycloalkyl group and denote its position on the parent chain with the number.
- The alkyl groups or cycloalkyl groups are written in alphabetical order.
(k)
Interpretation:
The systematic name for the following compound should be determined.
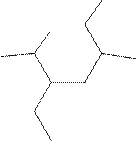
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY LL BUNDLE
- The predominant components of our atmosphere are N₂, O₂, and Ar in the following mole fractions: χN2 = 0.780, χO2 = 0.21, χAr = 0.01. Assuming that these molecules act as ideal gases, calculate ΔGmix, ΔSmix, and ΔHmix when the total pressure is 1 bar and the temperature is 300 K.arrow_forwarddG = Vdp - SdT + μA dnA + μB dnB + ... so that under constant pressure and temperature conditions, the chemical potential of a component is the rate of change of the Gibbs energy of the system with respect to changing composition, μJ = (∂G / ∂nJ)p,T,n' Using first principles prove that under conditions of constant volume and temperature, the chemical potential is a measure of the partial molar Helmholtz energy (μJ = (∂A / ∂nJ)V,T,n')arrow_forwardThe vapor pressure of dichloromethane at 20.0 °C is 58.0 kPa and its enthalpy of vaporization is 32.7 kJ/mol. Estimate the temperature at which its vapor pressure is 66.0 kPa.arrow_forward
- Draw the structure of A, the minor E1 product of the reaction. Cl Skip Part Check F1 esc CH_CH OH, D 3 2 Click and drag to start drawing a structure. 80 R3 F4 F2 F3 @ 2 # $ 4 3 Q W 95 % KO 5 F6 A F7 × G ☐ Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ►II A A F8 F9 F10 FL 6 7 88 & * 8 9 LLI E R T Y U A S D lock LL F G H 0 P J K L Z X C V B N M 9 Harrow_forwardFrom the choices given, which two substances have the same crystal structure? (Select both) Group of answer choices ZnS (zincblende) Diamond TiO2 (rutile) ZnS (wurtzite)arrow_forwardPotassium (K) blends with germanium (Ge) to form a Zintl phase with a chemical formula of K4Ge4. Which of the following elements would you expect potassium to blend with to form an alloy? Electronegativities: As (2.0), Cl (3.0), Ge (1.8), K (0.8), S (2.5), Ti (1.5) Group of answer choices Arsenic (As) Sulfur (S) Chlorine (Cl) Titanium (Ti)arrow_forward
- Consider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forwardHow many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forwardV Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forward
- Consider the reaction below and answer the following questions. Part 1 of 4 Br NaOCH2CH3 Identify the mechanisms involved. Check all that apply. SN 1 SN 2 E1 E2 None of the above Part 2 of 4 Skip Part Check esc F1 F2 lock 1 2 Q W A S #3 80 F3 F4 F5 F6 Save For © 2025 McGraw Hill LLC. All Rights Reserved. Terms ˇˇ % & 4 5 6 89 7 IK A 分 བ F7 F8 F9 F * E R T Y U 8 9 D F G H K V B N M 0 Oarrow_forwardWhat kind of holes are not generated when solid-state particles adopt a close packing pattern? Group of answer choices tetrahedral cubic octahedral None of the other choices are correctarrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forward

 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning




