
Write a net ionic equation for any precipitation reaction that occurs when 1 M solutions of the following are mixed.
(a) copper(II) sulfate and sodium chloride
(b) manganese(II) nitrate and ammonium hydroxide
(c) silver nitrate and hydrochloric acid
(d) nickel(II) sulfate and potassium hydroxide
(e) ammonium carbonate and sodium nitrate
(a)
Interpretation:
The net ionic equation should be written when copper(II) sulfate and sodium chloride are mixed.
Concept introduction:
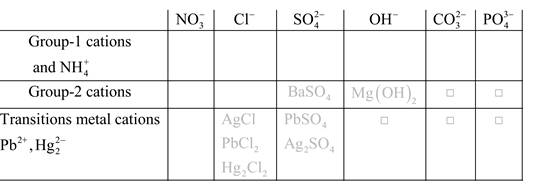
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
No precipitation occurs.
Explanation of Solution
Copper(II) sulfate:
Sodium chloride:
Reaction for the solution of copper(II) sulfate and sodium chloride is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
(b)
Interpretation:
The net ionic equation should be written when manganese(II) nitrate and ammonium hydroxide are mixed.
Concept introduction:
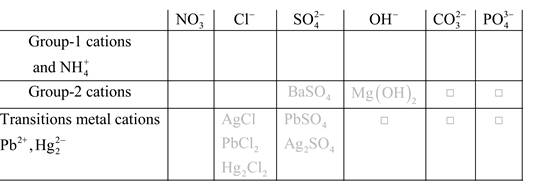
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Manganese(II) nitrate:
Ammonium hydroxide:
Reaction for the solution of manganese(II) nitrate and ammonium hydroxide is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(c)
Interpretation:
The net ionic equation should be written when silver nitrate and hydrochloric acid are mixed.
Concept introduction:
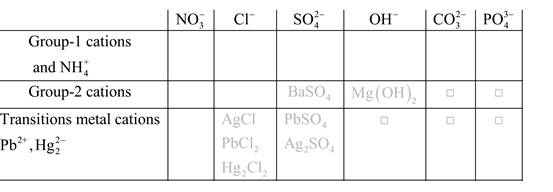
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Silver nitrate:
Hydrochloric acid:
Reaction for the solution of silver nitrate and hydrochloric acid is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(d)
Interpretation:
The net ionic equation should be written when nickel(II) sulfate and potassium hydroxide are mixed.
Concept introduction:
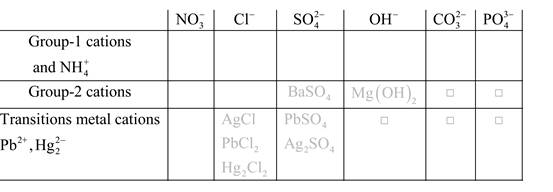
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Nickel(II) sulfate:
Potassium hydroxide:
Reaction for the solution of silver nitrate and hydrochloric acid is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(e)
Interpretation:
The net ionic equation should be written when ammonium carbonate and sodium nitrate are mixed.
Concept introduction:
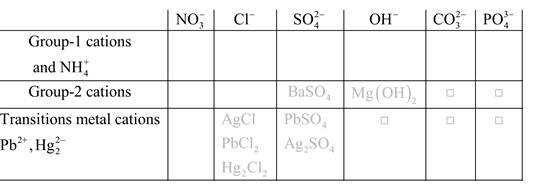
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
No precipitation occurs.
Explanation of Solution
Ammonium carbonate:
Sodium nitrate:
Reaction for the solution of silver nitrate and hydrochloric acid is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
Want to see more full solutions like this?
Chapter 4 Solutions
CHEMISTRY:PRIN.+REACTIONS-OWLV2 ACCESS
- Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forward
- Briefly state the electrocapillary equation for ideally polarized electrodes.arrow_forwardWhat is surface excess according to the Gibbs model?arrow_forwardUsing Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forward
- The molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





