
(a)
Interpretation:
Chair conformation of given compounds should be drawn and most stable compound is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:
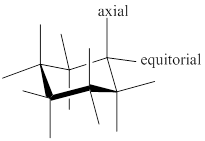
Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(b)
Interpretation:
Chair conformation of given compounds should be drawn and most stable compound is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(c)
Interpretation:
Chair conformation of given compounds should be drawn and most stable compound is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
(d)
Interpretation:
Chair conformation of given compounds should be drawn and most stable compound is needed to be predicted between them.
Concept introduction:
Chair conformer: chair conformer is a stable conformer for cyclohexane compound. In this chair conformer two positions are important for substitutions one is equatorial and other one axial position. Axial positions are parallel to the axis of ring while equatorial positions are perpendicular to the axis of the ring.
Example:

Stereochemistry conversions in chair conformation are shown below.
| Substituent position | cis | trans |
| 1,2 position | (a,e) or (e,a) | (a,a) or (e,e) |
| 1,3 position | (a,a) or (e,e) | (a,e) or (e,a) |
| 1,4 position | (a,e) or (e,a) | (a,a) or (e,e) |
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
- 19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forwardIndicate the product of the reaction OH OH CH3-CC- Ph + H2SO4 a 20°C | CH3 Pharrow_forward
- 35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forwardpls helparrow_forward
- 27) Draw the energy level diagram and write the full and shorthand electron configuration for a neutral sulfur atom.arrow_forwardIndicate whether these compounds are isomers, enantiomers, or tautomers. OCH OCH محمد ممدarrow_forward30) Substance A to E below are listed with several of their properties. The identities of the substances are identified in random order below: Iron, ethane, ethanol, sodium nitrate, graphite First classify each substance as either a polar covalent compound, non-polar covalent compound, ionic compound, metallic solid, or network solid. Write your predictions in the sixth coloumn of the chart, under "type of substance." Then, identify the identity of the substance in the last coloumn. Substance Melting Point Boiling Point Solubility in H₂O Electrical Conductivity Type of Substance Identity of Substance (°C) (°C) as: Solid, Liquids, Solution A -182 -88 Insoluble No/No/- B 1538 2862 Insoluble Yes/Yes/- C 308 380 Soluble Yes/Yes/Yes Ꭰ 3456 Insoluble No/-/- E -114 78 Soluble No/No/Noarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





