
FUND OF THERMODYNAMICS- UND CUSTOM
2nd Edition
ISBN: 9781119694205
Author: Borgnakke
Publisher: WILEY C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.89P
A
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Problem 19: Determine the force in members HG, HE, and DE of the truss, and state if the
members are in tension or compression.
4 ft
K
J
I
H
G
B
C
D
E
F
-3 ft
-3 ft 3 ft 3 ft 3 ft-
1500 lb 1500 lb 1500 lb 1500 lb 1500 lb
Problem 14: Determine the reactions at the pin A, and the tension in cord. Neglect the
thickness of the beam.
F1=26kN
F2
13
12
80°
-2m
3m
Problem 22: Determine the force in members GF, FC, and CD of the bridge truss and state
if the members are in tension or compression.
F
15 ft
B
D
-40 ft
40 ft
-40 ft
40 ft-
5 k
10 k
15 k
30 ft
E
Chapter 4 Solutions
FUND OF THERMODYNAMICS- UND CUSTOM
Ch. 4 - A temperature difference drives a heat transfer...Ch. 4 - What is the effect can be felt upstream in a flow?Ch. 4 - Prob. 4.3PCh. 4 - Air at 500 kPa is expanded to l00 kPa in two...Ch. 4 - A windmill takes out a fraction of the wind...Ch. 4 - An underwater turbine extracts a fraction of the...Ch. 4 - A liquid water turbine at the bottom of a dam...Ch. 4 - You blow a balloon up with air. What kinds of work...Ch. 4 - Storage tanks of cryogenic liquids (O2,N2,CH4) are...Ch. 4 - A large brewery has a pipe of cross-sectional area...
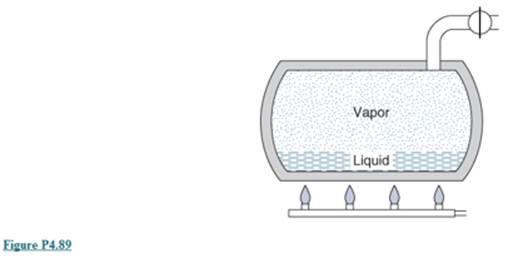
Ch. 4 - A pool is to be filled with 60m3 water from a...Ch. 4 - Natural gas, CH4 , flowing in a 5cm -diameter pipe...Ch. 4 - A boiler receives a constant flow of 5000kg/h...Ch. 4 - A 0.6m -diameter household fan takes air in at...Ch. 4 - Liquid water at 15°C flows out of nozzle straight...Ch. 4 - A nozzle receives an ideal gas flow with a...Ch. 4 - In a jet engine a flow afar at 1000K,200kPa, and...Ch. 4 - The wind is blowing horizontally at 30m/s in a...Ch. 4 - A meteorite hits the upper atmosphere at 3000m/s ,...Ch. 4 - Carbon dioxide is throttled from 20C,2000kPa to...Ch. 4 - Saturated liquid R-410A at 25°C is throttled to...Ch. 4 - Carbon dioxide used as a natural refrigerant flows...Ch. 4 - Liquid water at 180C,2000kPa is throttled into a...Ch. 4 - Methane at 1MPa,250K is throttled through a valve...Ch. 4 - A steam turbine has an n1et of 3kg/s water at 1200...Ch. 4 - Air at 20m/s,1500K,875kPa with 5kg/s flows into a...Ch. 4 - Solve the previous problem using Table A.7.Ch. 4 - A wind turbine can extract at most a fraction...Ch. 4 - A liquid water turbine receives 2kg/s water at...Ch. 4 - A small high-speed turbine operating on compressed...Ch. 4 - Hoover Dam across the Colorado River dams up Lake...Ch. 4 - What is the specific work one can get from Hoover...Ch. 4 - R-410A in a commercial refrigerator flows into the...Ch. 4 - A compressor brings nitrogen from 100kPa,290K to...Ch. 4 - A refrigerator uses the natural refrigerant carbon...Ch. 4 - A factory generates compressed air from l00kPa,17C...Ch. 4 - A compressor brings R-134a from...Ch. 4 - An exhaust fan in a building should be able to...Ch. 4 - Prob. 4.39PCh. 4 - The air conditioner in a house or a car has a...Ch. 4 - A boiler section boils 3kg/s saturated liquid...Ch. 4 - A superheater takes 3kg/s saturated water vapor in...Ch. 4 - Carbon dioxide enters a steady-state, steady-flow...Ch. 4 - Find the heat transfer in Problem 4.13.Ch. 4 - A chiller cools liquid water for air-conditioning...Ch. 4 - Saturated liquid nitrogen at 600 kPa enters a...Ch. 4 - Prob. 4.47PCh. 4 - Liquid nitrogen at 90K,400kPa flows into a probe...Ch. 4 - Liquid glycol flows around an engine, cooling it...Ch. 4 - An irrigation pump takes water from a river at...Ch. 4 - A pipe from one building to another flows water at...Ch. 4 - A river flowing at 0.5m/s across a 1-m-high and...Ch. 4 - A cutting tool uses a nozzle that generates a...Ch. 4 - An adiabatic steam turbine in a power plant...Ch. 4 - Prob. 4.55PCh. 4 - A steam turbine receives steam from two boilers...Ch. 4 - Prob. 4.57PCh. 4 - Prob. 4.58PCh. 4 - A condenser (heat exchanger) brings 1kg/s water...Ch. 4 - Steam at 500kPa,300C is used to heat cold water at...Ch. 4 - A dual-fluid heat exchanger has 5kg/s water...Ch. 4 - An energy recovery heat exchanger, shown in Fig....Ch. 4 - Do the previous problem if the water is heated to...Ch. 4 - In a co-flowing (same-direction) heat exchanger,...Ch. 4 - An a water counter flowing heat exchanger has one...Ch. 4 - An automotive radiator has glycol at 95°C enter...Ch. 4 - Prob. 4.67PCh. 4 - Two air flows are combined to a single flow. One...Ch. 4 - An open feedwater heater in a power plant heats...Ch. 4 - A de-superheater has a flow of ammonia of 1.5kg/s...Ch. 4 - A mixing chamber with heat transfer receives 2kg/s...Ch. 4 - A geothermal supply of hot water at 500kPa,150C is...Ch. 4 - A flow of 5kg/s water at l00kPa,20C should be...Ch. 4 - A two-stage compressor takes nitrogen ri at...Ch. 4 - The intercooler in the previous problem uses cold...Ch. 4 - Prob. 4.76PCh. 4 - A modern jet engine has a temperature after...Ch. 4 - A proposal is made to use a geothermal supply of...Ch. 4 - Prob. 4.79PCh. 4 - An initially empty canister of volume 0.2m3 is...Ch. 4 - Repeat the previous problem but use the line...Ch. 4 - A tank contains 1m3 air at 100kPa,300K . A pipe...Ch. 4 - A 2.5L tank initially is empty, and we want to...Ch. 4 - An insulated 2m3 tank is to be charged with R-134a...Ch. 4 - Repeat the previous problem if the valve is closed...Ch. 4 - A 3m3 ? cryogenic storage tank contains nitrogen...Ch. 4 - A nitrogen line at 300K,0.5MPa , shown in Fig....Ch. 4 - Prob. 4.88PCh. 4 - A 200L tank (see Fig. P4.89) initially contains...Ch. 4 - A 1-L can of R-410A is at room temperature, 20°C,...Ch. 4 - Steam at 3MPa,400C enters a turbine with a...Ch. 4 - In a glass factory a 2m -wide sheet of glass at...Ch. 4 - Assume a setup similar to that of the previous...Ch. 4 - Three a flows, all at 200 kPa, e connected to the...Ch. 4 - A 1m3,40kg rigid steel tank contains air at 500...Ch. 4 - An insulated spring-loaded piston/cylinder device,...Ch. 4 - A piston/cyl. setup like Fig. 4.96 is such that at...Ch. 4 - A mass-loaded piston/cylinder shown in Fig. P4.98,...Ch. 4 - A flow of 2kg/s of water at 500kPa,20C is heated...Ch. 4 - Refrigerant R-410A at l00psia,60F flows at...Ch. 4 - A pool is to be filled with 2500ft3 water from a...Ch. 4 - Liquid water at 60 F flows out of a nozzle...Ch. 4 - Prob. 4.103EPCh. 4 - Prob. 4.104EPCh. 4 - Nitrogen gas flows into a convergent nozzle at...Ch. 4 - A meteorite hits the upper atmosphere at 10000ft/s...Ch. 4 - Refrigerant R-410A flows out of a cooler at...Ch. 4 - Saturated vapor R-410A at 75 psia is throttled to...Ch. 4 - A wind turbine can exact at most a fraction 16/27...Ch. 4 - A liquid water turbine receives 4Ibm/s water at...Ch. 4 - Prob. 4.111EPCh. 4 - What is the specific work one can get from Hoover...Ch. 4 - A small-speed turbine operating on compressed air...Ch. 4 - R.410A in a commercial refigerator flows into the...Ch. 4 - An exhaust fan in a building should be able to...Ch. 4 - Carbon dioxide gas enters a steady-state,...Ch. 4 - Prob. 4.117EPCh. 4 - Liquid glycol flows around an engine, cooling t as...Ch. 4 - Prob. 4.119EPCh. 4 - Prob. 4.120EPCh. 4 - Do the previous problem if the water is just...Ch. 4 - A dual-fluid heat exchanger has l0Ibm/s water...Ch. 4 - Steam at 80psia,600F is used to heat cold water at...Ch. 4 - Prob. 4.124EPCh. 4 - Two flows of air are both at 30 psia one has...Ch. 4 - A de-superheater has a flow of ammonia of 3Ibm/s...Ch. 4 - A two-stage compressor takes nitrogen n at...Ch. 4 - The intercooler in the previous problem uses cold...Ch. 4 - Prob. 4.129EPCh. 4 - Prob. 4.130EPCh. 4 - A tank contains l0ft3 of air at 15psia,540R . A...Ch. 4 - Prob. 4.132EPCh. 4 - In a glass factory a 6 ft-wide sheet of glass at...Ch. 4 - A mass-loaded piston/cylinder containing air is at...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Problem 20: Determine the force in members BC, HC, and HG. After the truss is sectioned use a single equation of equilibrium for the calculation of each force. State if the members are in tension or compression. 5 kN 4 kN 4 kN 3 kN 2 kN B D E F 3 m -5 m- -5 m- 5 m 5 m-arrow_forwardAn experimental setup is being built to study the flow in a large water main (i.e., a large pipe). The water main is expected to convey a discharge (Qp). The experimental tube will be built at a length scale of 1/20 of the actual water main. After building the experimental setup, the pressure drop per unit length in the model tube (APm/Lm) is measured. Problem (19): Given the value of Qp [m³/s], and assuming Reynolds number similitude between the water main and experimental tube, calculate the flow rate in the model tube (Qm) in [lit/s]. = 30.015 m^3/sarrow_forwardProblem 11: The lamp has a weight of 15 lb and is supported by the six cords connected together as shown. Determine the tension in each cord and the angle 0 for equilibrium. Cord BC is horizontal. E 30° B 60° Aarrow_forward
- Problem 10: If the bucket weighs 50 lb, determine the tension developed in each of the wires. B $30° 5 E D 130°arrow_forwardProblem 3: Four-Force Equilibrium Knowing the forces in members A and C, determine the force of B and D, assuming the system is in equilibrium. A structural joint is held in equilibrium by four forces acting along different members. • Member A applies a force of 4 kN at an angle of 60° above the positive x-axis. • Member C applies a force of 2 kN horizontally to the left along the x-axis. • Member B applies an unknown force along the horizontal direction. • Member D applies an unknown force at an angle of 45° above the negative x-axis. Determine the forces in members B and D, assuming the system is in static equilibrium. 4 kN 2 kN C 45° A D 60° FB Barrow_forwardProblem 18: Determine the force in each member of the truss. State if the members are in tension or compression. 3 ft 3 ft 3 ft B D 4 ft 4 ft. 130 lb Earrow_forward
- Problem 16: Determine the force in each of the member of the truss and state if the members are in tension or compression. Set P₁ = 10 kN, P2 = 8 kN. 2 m G F E A A 1 m B 2 m 1 m P1 Darrow_forwardProblem 7: Determine the force in each cord for equilibrium of the 60-kg bucket. D E 4 m 4 m B 3 m- 3 m- 3 m.arrow_forwardProblem 15: Determine the reactions at the pin A and the tension in cord BC. Set F = 40 kN. Neglect the thickness of the beam. 26 kN F 13 12 -2 m 4 m B 4arrow_forward
- Problem 21: Determine the force in members EF, CF, and BC and state if the members are in tension or compression. 1.5 m 4 kN E D 8 kN B 2 m 2 marrow_forwardProblem 8: If the cords suspend the two buckets in the equilibrium position, determine the weight of bucket B. Bucket A has a weight of 60 lb. E 65° C 20° 40° F B 20° Aarrow_forwardProblem 4: Four Force Equilibrium The members of a truss are connected at joint O. Determine the magnitudes of F₁ and F2 for equilibrium, assuming 0 = 60°. 5 kN 7 kN 70° 30°arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
What is entropy? - Jeff Phillips; Author: TED-Ed;https://www.youtube.com/watch?v=YM-uykVfq_E;License: Standard youtube license