
Study Guide And Selected Solutions Manual For Chemistry Format: Paperback
13th Edition
ISBN: 9780134553986
Author: Timberlake, Karen C
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4, Problem 4.87UTC
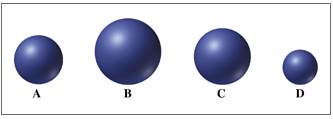
Match the spheres A through D with atoms of Li, Na, K, and Rb. (4.7)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name the molecules & Identify any chiral center
CH3CH2CH2CHCH₂CH₂CH₂CH₂
OH
CH₂CHCH2CH3
Br
CH3
CH3CHCH2CHCH2CH3
CH3
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Chapter 4 Solutions
Study Guide And Selected Solutions Manual For Chemistry Format: Paperback
Ch. 4.1 - Write the symbols for the following elements. a....Ch. 4.1 - Prob. 4.2PPCh. 4.1 - 4.3 Write the name for the symbol of each of the...Ch. 4.1 - Prob. 4.4PPCh. 4.1 - Prob. 4.5PPCh. 4.1 - Prob. 4.6PPCh. 4.2 - 4.7 Identify the group or period number described...Ch. 4.2 - 4.8 Identify the group or period number described...Ch. 4.2 - Prob. 4.9PPCh. 4.2 - 4.10 Give the symbol of the element described by...
Ch. 4.2 - Prob. 4.11PPCh. 4.2 - Identify each of the following elements as a...Ch. 4.2 - Prob. 4.13PPCh. 4.2 - 4.14 Using TABLE 4.4, identify the function of...Ch. 4.2 - Prob. 4.15PPCh. 4.2 - Prob. 4.16PPCh. 4.3 - Identify each of the following as describing...Ch. 4.3 - 4.18 Identify each of the following as describing...Ch. 4.3 - What did Rutherford determine about the structure...Ch. 4.3 - 4.20 How did Thomson determine that the electrons...Ch. 4.3 - Prob. 4.21PPCh. 4.3 - Is each of the following statements true or false?...Ch. 4.3 - Prob. 4.23PPCh. 4.3 - 4.24 Sometimes clothes cling together when...Ch. 4.4 - 4.25 Would you use the atomic number, mass...Ch. 4.4 - 4.26 Identify the type of subatomic particles...Ch. 4.4 - Prob. 4.27PPCh. 4.4 - Prob. 4.28PPCh. 4.4 - Prob. 4.29PPCh. 4.4 - Prob. 4.30PPCh. 4.4 - Complete the following table for atoms of...Ch. 4.4 - Prob. 4.32PPCh. 4.5 - What are the number of protons, neutrons, and...Ch. 4.5 - 4.34 What are the number of protons, neutrons, and...Ch. 4.5 - Write the atomic symbol for the isotope with each...Ch. 4.5 - Prob. 4.36PPCh. 4.5 - Argon has three naturally occurring isotopes, with...Ch. 4.5 - Strontium has four naturally occurring isotopes,...Ch. 4.5 - Prob. 4.39PPCh. 4.5 - 4.40 Two isotopes of rubidium occur naturally, ...Ch. 4.5 - Prob. 4.41PPCh. 4.5 - Prob. 4.42PPCh. 4.5 - There are two naturally occurring isotopes of...Ch. 4.5 - There are five naturally occurring isotopes of...Ch. 4.6 - Electrons can move to higher energy levels when...Ch. 4.6 - Prob. 4.46PPCh. 4.6 - Prob. 4.47PPCh. 4.6 - Prob. 4.48PPCh. 4.6 - Prob. 4.49PPCh. 4.6 - 4.50 Write the electron arrangement for each of...Ch. 4.6 - Prob. 4.51PPCh. 4.6 - Prob. 4.52PPCh. 4.7 - What is the group number and number of valence...Ch. 4.7 - 4.54 What is the group number and number of...Ch. 4.7 - Prob. 4.55PPCh. 4.7 - Write the group number and draw the Lewis symbol...Ch. 4.7 - Prob. 4.57PPCh. 4.7 - Prob. 4.58PPCh. 4.7 - Prob. 4.59PPCh. 4.7 - Prob. 4.60PPCh. 4.7 - Prob. 4.61PPCh. 4.7 - 4.62 Select the element in each pair with the...Ch. 4.7 - Prob. 4.63PPCh. 4.7 - Prob. 4.64PPCh. 4.7 - Prob. 4.65PPCh. 4.7 - Prob. 4.66PPCh. 4.7 - Prob. 4.67PPCh. 4.7 - Prob. 4.68PPCh. 4.7 - Prob. 4.69PPCh. 4.7 - 4.70 Fill in the following blanks using higher or...Ch. 4.7 - Prob. 4.71PPCh. 4.7 - Prob. 4.72PPCh. 4.7 - Prob. 4.73PPCh. 4.7 - 4.74 Which statements completed with a to e will...Ch. 4.7 - a. What is the group number and name of the group...Ch. 4.7 - a. How many neutrons are in K-41? b. Write the...Ch. 4 - Prob. 4.77UTCCh. 4 - Use Rutherford’s gold-foil experiment to answer...Ch. 4 - Prob. 4.79UTCCh. 4 - Prob. 4.80UTCCh. 4 - Prob. 4.81UTCCh. 4 - Prob. 4.82UTCCh. 4 - Prob. 4.83UTCCh. 4 - Prob. 4.84UTCCh. 4 - Prob. 4.85UTCCh. 4 - Prob. 4.86UTCCh. 4 - Match the spheres A through D with atoms of Li,...Ch. 4 - Prob. 4.88UTCCh. 4 - Prob. 4.89UTCCh. 4 - Of the elements Sn, Xe, Te, Sr, I, and Rb,...Ch. 4 - Prob. 4.91APPCh. 4 - Prob. 4.92APPCh. 4 - Prob. 4.93APPCh. 4 - Prob. 4.94APPCh. 4 - Prob. 4.95APPCh. 4 - Prob. 4.96APPCh. 4 - Prob. 4.97APPCh. 4 - Write the name and symbol of the element with the...Ch. 4 - Prob. 4.99APPCh. 4 - Prob. 4.100APPCh. 4 - Prob. 4.101APPCh. 4 - Prob. 4.102APPCh. 4 - Prob. 4.103APPCh. 4 - Prob. 4.104APPCh. 4 - Prob. 4.105APPCh. 4 - Prob. 4.106APPCh. 4 - Prob. 4.107APPCh. 4 - Prob. 4.108APPCh. 4 - Why is the ionization energy of Ca higher than K,...Ch. 4 - Prob. 4.110APPCh. 4 - Prob. 4.111APPCh. 4 - Of the elements F, Br, Cl, and I, which (4.7) a....Ch. 4 - Prob. 4.113CPCh. 4 - Prob. 4.114CPCh. 4 - Prob. 4.115CPCh. 4 - Prob. 4.116CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY