
Concept explainers
(a)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(a)
Explanation of Solution
Given molecule,
Increase in
(b)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(b)
Explanation of Solution
Given molecule,
Increase in
(c)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(c)
Explanation of Solution
Given molecule,
Increase in
(d)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(d)
Explanation of Solution
Given molecule,
Increase in
(e)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(e)
Explanation of Solution
Given molecule,
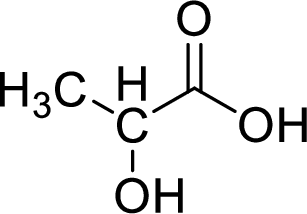
Increase in
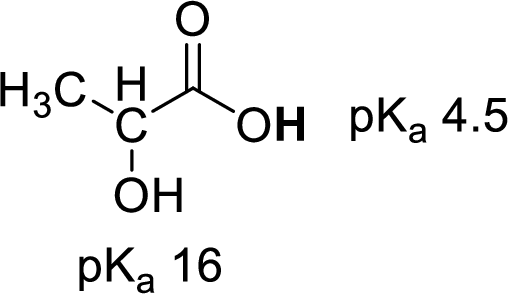
(f)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(f)
Explanation of Solution
Given molecule,
Increase in
(g)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(g)
Explanation of Solution
Given molecule,
Increase in
(h)
Interpretation:
The most acidic hydrogen in given molecule has to be labelled and justify it using
Concept Introduction:
Position of equilibrium in acid-base reaction:
In an acid-base reaction, the position of equilibrium always favors reaction of the stronger acid and stronger base to form the weaker acid and weaker base. Therefore, the major species present at equilibrium in an acid-base reaction are weaker acid and weaker base. The reaction equilibrium shifts to a direction where weaker acid and weaker base is formed. Acids with greater
(h)
Explanation of Solution
Given molecule,
Increase in
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry, Loose-leaf Version
- Which of the following are descriptions of possible starting material for this reaction? H ? trace acid an ester a ketone an imine an aldehyde a carboxylic acid an enamine a primary amine a secondary amine a tertiary aminearrow_forwardNonearrow_forwardWhat are the reagents needed for this and the third structure I only got the top right structure rightarrow_forward
