
To find how formal charges are used to choose between possible molecular structures.
Explanation of Solution
1) Concept :
A Formal charge (FC) is not a real charge to calculate the charge of an individual atom in a molecule or polyatomic ion. It is used to assign the number of electrons to an atom in a structure.
2) Formula:
Using this formula FC on an atom in a molecule or polyatomic ion can be calculated.
3) Calculation:
Let’s take the example of N2O molecule. The various resonance structures possible for N2O molecule are:
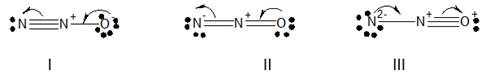
In structure (I),
Therefore, the sum of FC on three atoms = (0) + (+1) + (-1) = 0
In structure (II),
Therefore, the sum of FC on three atoms = (-1) + (+1) + (0) = 0
In structure (III),
Therefore, the sum of FC on three atoms = (-2) + (+1) + (+1) = 0
Now to decide the best or most contributing resonance structures, some rules or criteria have to be followed.
Criteria to choose best molecular structure
a) The best structure shall be one in which each atom has formal charge equal to zero.
b) If this is not possible, then structure having most of the atoms carrying zero or nearly zero charge shall be considered.
c) Negative charge should reside on more electronegative element(s)
Considering these three criteria, it can be concluded that, among the structures I, II and III, structure (I) is best structure to represent N2O molecule.
Structure (III) is least contributing structure and (II) can be considered as the intermediate between (I) and (III).
Conclusion:
To choose the best resonance structure in a molecule or in a polyatomic ion, the above three criteria should be followed.
Want to see more full solutions like this?
Chapter 4 Solutions
CHEM:ATOM FOC 2E CL (TEXT)
- 6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forwardDraw the correct productsarrow_forward
- E Organic Chemistry Maxwell Draw the correct products, in either order, for the ozonolysis reaction: 1) O3, CH2Cl2, -78 °C Product 1 + Product 2 2) Zn, HOAc Draw product 1. Select Draw Templates More C H O presented by M Draw product 2. Erase Select Draw Templates M / # # carrow_forward✓ edict the products of this organic reaction: ---- ။ A CH3–C−NH–CH2–C−CH3 + KOH ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibiliarrow_forwardPredict the product of this organic reaction: A HO-C-CH3 + CH3NH2 P+ H2O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. marrow_forward
- H 1) OsO4, pyridine 2) Na2SO3 or NaHSO3 in H₂O 2 productsarrow_forward● Biological Macromolecules Naming and drawing cyclic monosaccharides Your answer is incorrect. • Row 1: Your answer is incorrect. Row 3: Your answer is incorrect. • Row 4: Your answer is incorrect. Try again... 0/5 Give the complete common name, including anomer and stereochemistry labels, of the following molecules. You will find helpful information in the ALEKS resource. CH2OH OH OH H H I H OH OH H] H CH2OH H OH ẞ-L-sorbose HOCH2 OH OH H HOCH2 H OH OH H OH H H CH2OH OH H H OH H I- H OH H OH Explanation Recheck W E R % 25 α B Y X & 5 D F G H McGraw Hill LLC. All Rights Reserved. Terms of Use | Pr Parrow_forwardWhat is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- Stuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forwardPredict the product of this organic reaction: + NH2 HO A P+ H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Click and drag to start drawing a structure. ✓arrow_forward个 Stuc X ctclix ALE X A ALE × A ALE X Lab x (195 × Nut x M Inbx EF 目 → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpz Chapter 12 HW = Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited Part: 1/2 Part 2 of 2 Give the IUPAC name. Check 3 50°F Clear ©2025 McGraw Hill L Q Search webp a عالياكarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





