
Concept explainers
Interpretation:
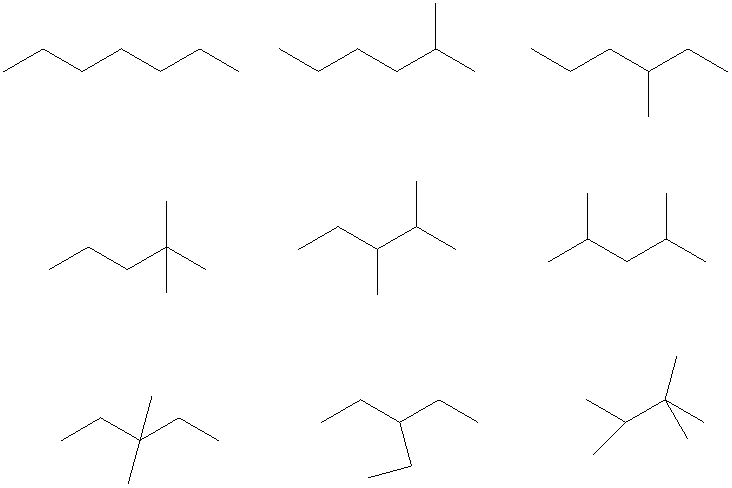
The condensed and bond-line structural formula for all the constitutional isomers with molecular formula
Concept introduction:
Constitutional isomers are the isomers in which the molecular formula remains same but the arrangements of groups or atoms in the structure are different.
Mostly organic compound is represented by the bond line structural formulas.
In bond line formula each bond is represented by a line.
At the end of each line if no atom is shown than a carbon atom is present.
Condensed structural formula is defined as the formula in which all the carbon-carbon bonds are eliminated and the hydrogen atom written just after the carbon atom.
Answer to Problem 1PP
Solution:
Bond-line structural formula:
Condensed formula:
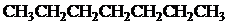
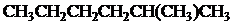
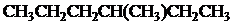
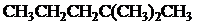
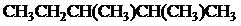
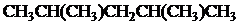
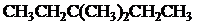
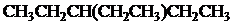
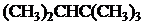
Explanation of Solution
Given: The molecular formula is
In

All the above structures have same molecular formula,
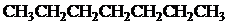
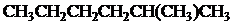
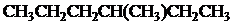
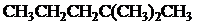
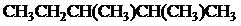
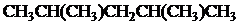
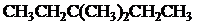
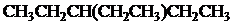
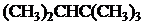
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEMISTRY-ETEXT REG ACCESS
- Can I please get help with identifying these?arrow_forward4. Calculate the pH of a 0.10 M acetic acid (CH3COOH) solution if the Ka of acetic acid = 1.8 x 10-5arrow_forwardDraw the Zaitsev product of the dehydration of this alcohol. + I X 5 OH ざ~ TSOH Click and drag to start drawing a structure.arrow_forward
- Please help with identifying these.arrow_forwardFor the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forwardCan I please get this answered? With the correct number of significant digits.arrow_forward
- Draw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forwardCan I please get help with this graph, if you could show exactly where it needs to pass through please.arrow_forwardDraw the condensed structure of 1,3-dihydroxy-2-pentanone. Explanation Check Click anywhere to draw the first atom of your structure. Х C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of use +arrow_forward
- 0.500 moles of NOCl are placed into a 1.00 L vessesl at 700K and after the system comes to equilibrium, the consentration of NOCl is 0.440 M. Calculate the equilibrium constant Kc for the reaction: 2NOCL (g) --> 2NO (g) + Cl2 (g)arrow_forwardWhat is the hydronium ion concentration in a solution of water that has a hydroxide ion concentrationof 1.0 x 10-2 M?arrow_forwardIdentify conjugate acid-base pairs in the following reactions:HBr (aq) + H2O (l) ⇌ H3O+ (aq) + Br- (aq) - OH (aq) + CH3COOH (aq) ⇌ H2O (l) + CH3COO- (aq)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
