
Campbell Biology
12th Edition
ISBN: 9780135188743
Author: Urry
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.1, Problem 3CC
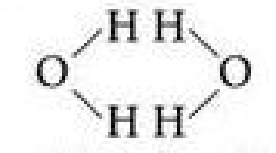
Why is it unlikely that two neighboring water molecules would be arranged like this?
Expert Solution & Answer
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

schedule01:15
Students have asked these similar questions
Joden Koepp
olor in chickens is due to incomplete dominance. BB = Black chicken, WW = White
BLOOD TYPES
Arhite chicken is
In humans, Rh positive blood is dominant (R) over Rh negative blood (r). A man with type 0, Rh positive
blood (whose mother had Rh negative blood), marries a woman with type AB, Rh negative blood. Several
children were born.
is?
R
R
Genotypes
Phenotypes
RRR
RR Rr
Rr
4/16 RR
R RR RK Rr
Rr
4/16 rr
3/4 Rh posi
1/4 Rh negu
1/2 Rr
rr
rr
rrrr
88
888
75
e genotype of the man?
the genotype of the woman?
The mother of the man had type AB blood.
Please indentify the unknown organism
Please indentify the unknown organism
Chapter 3 Solutions
Campbell Biology
Ch. 3.1 - MAKE CONNECTIONS What is electronegativity, and...Ch. 3.1 - WHAT IF? What would be the effect on the...Ch. 3.1 - Why is it unlikely that two neighboring water...Ch. 3.1 - WHAT IF? What would be the effect on the...Ch. 3.2 - Prob. 1CCCh. 3.2 - Prob. 2CCCh. 3.2 - WHAT IF? A water strider (an insect that can walk...Ch. 3.2 - WHAT IF? A water strider (an insect that can walk...Ch. 3.2 - Prob. 5CCCh. 3.3 - Compared with a basic Solution at pH 9, the same...
Ch. 3.3 - HCl is a strong acid that dissociates in water:...Ch. 3.3 - Prob. 3CCCh. 3.3 - Prob. 4CCCh. 3 - DRAW IT Label a hydrogen bond and a polar...Ch. 3 - Describe how different types of solutes dissolve...Ch. 3 - Explain what happens to the concentration...Ch. 3 - Which of the following is a hydrophobic material?...Ch. 3 - Prob. 2TYUCh. 3 - Measurements show that the pH of a particuiar lake...Ch. 3 - What is the hydroxide ion concentration of the...Ch. 3 - A slice of pizza has 500 kcal. If we could burn...Ch. 3 - DRAW IT Draw the hydration shells that form around...Ch. 3 - In agricultural areas, farmers pay close attention...Ch. 3 - MAKE CONNECTIONS What do climate change (see...Ch. 3 - EVOLUTION CONNECTION This chapter explains how the...Ch. 3 - Prob. 10TYUCh. 3 - WRITE ABOUT A THEME: ORGANIZATION Several emergent...Ch. 3 - SYNTHESIZE YOUR KNOWLEDGE How do cats drink?...
Additional Science Textbook Solutions
Find more solutions based on key concepts
35. Consider the reaction.
The graph shows the concentration of Br2 as a function of time.
a. Use the g...
Chemistry: Structure and Properties (2nd Edition)
What name is given to the zone of greatest seismic activity?
Applications and Investigations in Earth Science (9th Edition)
Thiols such as ethanethiol and propanethiol can be used to reduce vitamin K epoxide to vitamin KH2, but they re...
Organic Chemistry (8th Edition)
Where is transitional epithelium found and what is its importance at those sites?
Anatomy & Physiology (6th Edition)
In rabbits, chocolate-colored fur (w+) is dominant to white fur (w), straight fur (c+) is dominant to curly fur...
Genetic Analysis: An Integrated Approach (3rd Edition)
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Please indentify the unknown organismarrow_forwardPlease indentify the unknown organismarrow_forward5G JA ATTC 3 3 CTIA A1G5 5 GAAT I I3 3 CTIA AA5 Fig. 5-3: The Eco restriction site (left) would be cleaved at the locations indicated by the arrows. However, a SNP in the position shown in gray (right) would prevent cleavage at this site by EcoRI One of the SNPs in B. rapa is found within the Park14 locus and can be detected by RFLP analysis. The CT polymorphism is found in the intron of the Bra013780 gene found on Chromosome 1. The Park14 allele with the "C" in the SNP has two EcoRI sites and thus is cleaved twice by EcoRI If there is a "T" in that SNP, one of the EcoRI sites is altered, so the Park14 allele with the T in the SNP has only one EcoRI site (Fig. 5-3). Park14 allele with SNP(C) Park14 allele with SNPT) 839 EcoRI 1101 EcoRI 839 EcoRI Fig. 5.4: Schematic restriction maps of the two different Park14 alleles (1316 bp long) of B. rapa. Where on these maps is the CT SNP located? 90 The primers used to amplify the DNA at the Park14 locus (see Fig. 5 and Table 3 of Slankster et…arrow_forward
- From your previous experiment, you found that this enhancer activates stripe 2 of eve expression. When you sequence this enhancer you find several binding sites for the gap gene, Giant. To test how Giant interacts with eve, you decide to remove all of the Giant binding sites from the eve enhancer. What results do you expect to see with respect to eve expression?arrow_forwardWhat experiment could you do to see if the maternal gene, bicoid, is sufficient to form anterior fates?arrow_forwardYou’re curious about the effect that gap genes have on the pair-rule gene, evenskipped (eve), so you isolate and sequence each of the eve enhancers. You’re particularly interested in one of the enhancers, which is just upstream of the eve gene. Describe an experimental technique you would use to find out where this particular eve enhancer is active.arrow_forward
- For short answer questions, write your answers on the line provided. To the right is the mRNA codon table to use as needed throughout the exam. First letter U บบบ U CA UUCPhe UUA UCU Phe UCC UUG Leu CUU UAU. G U UAC TV UGCys UAA Stop UGA Stop A UAG Stop UGG Trp Ser UCA UCG CCU] 0 CUC CUA CCC CAC CAU His CGU CGC Leu Pro CCA CAA Gin CGA Arg CUG CCG CAG CGG AUU ACU AAU T AUC lle A 1 ACC Thr AUA ACA AUG Mot ACG AGG Arg GUU GCU GUC GCC G Val Ala GAC Asp GGU GGC GUA GUG GCA GCG GAA GGA Gly Glu GAGJ GGG AACASH AGU Ser AAA1 AAG Lys GAU AGA CAL CALUCAO CAO G Third letter 1. (+7) Use the table below to answer the questions; use the codon table above to assist you. The promoter sequence of DNA is on the LEFT. You do not need to fill in the entire table. Assume we are in the middle of a gene sequence (no need to find a start codon). DNA 1 DNA 2 mRNA tRNA Polypeptide C Val G C. T A C a. On which strand of DNA is the template strand (DNA 1 or 2)?_ b. On which side of the mRNA is the 5' end (left or…arrow_forward3. (6 pts) Fill in the boxes according to the directions on the right. Structure R-C R-COOH OH R-OH i R-CO-R' R R-PO4 R-CH3 C. 0 R' R-O-P-OH 1 OH H R-C-H R-N' I- H H R-NH₂ \H Name Propertiesarrow_forward4. (6 pts) Use the molecule below to answer these questions and identify the side chains and ends. Please use tidy boxes to indicate parts and write the letter labels within that box. a. How many monomer subunits are shown? b. Box a Polar but non-ionizable side chain and label P c. Box a Basic Polar side chain and label BP d. Box the carboxyl group at the end of the polypeptide and label with letter C (C-terminus) H H OHHO H H 0 HHO H-N-CC-N-C-C N-C-C-N-GC-OH I H-C-H CH2 CH2 CH2 H3C-C+H CH2 CH2 OH CH CH₂ C=O OH CH2 NH2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Macromolecules | Classes and Functions; Author: 2 Minute Classroom;https://www.youtube.com/watch?v=V5hhrDFo8Vk;License: Standard youtube license