
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
14th Edition
ISBN: 9780136873891
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.1, Problem 3.2.2PE
Balance these equations by providing the missing coefficients
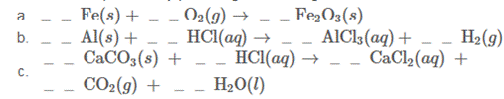
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
19.78 Write the products of the following sequences of reactions. Refer to your reaction road-
maps to see how the combined reactions allow you to "navigate" between the different
functional groups. Note that you will need your old Chapters 6-11 and Chapters 15-18
roadmaps along with your new Chapter 19 roadmap for these.
(a)
1. BHS
2. H₂O₂
3. H₂CrO4
4. SOCI₂
(b)
1. Cl₂/hv
2. KOLBU
3. H₂O, catalytic H₂SO4
4. H₂CrO4
Reaction
Roadmap
An alkene 5. EtOH
6.0.5 Equiv. NaOEt/EtOH
7. Mild H₂O
An alkane
1.0
2. (CH3)₂S
3. H₂CrO
(d)
(c)
4. Excess EtOH, catalytic H₂SO
OH
4. Mild H₂O*
5.0.5 Equiv. NaOEt/EtOH
An alkene 6. Mild H₂O*
A carboxylic
acid
7. Mild H₂O*
1. SOC₁₂
2. EtOH
3.0.5 Equiv. NaOEt/E:OH
5.1.0 Equiv. NaOEt
6.
NH₂
(e)
1. 0.5 Equiv. NaOEt/EtOH
2. Mild H₂O*
Br
(f)
i
H
An aldehyde
1. Catalytic NaOE/EtOH
2. H₂O*, heat
3. (CH,CH₂)₂Culi
4. Mild H₂O*
5.1.0 Equiv. LDA
Br
An ester
4. NaOH, H₂O
5. Mild H₂O*
6. Heat
7.
MgBr
8. Mild H₂O*
7. Mild H₂O+
Li+ is a hard acid. With this in mind, which if the following compounds should be most soluble in water?
Group of answer choices
LiBr
LiI
LiF
LiCl
Q4: Write organic product(s) of the following reactions and show the curved-arrow mechanism
of the reactions.
Br
MeOH
OSO2CH3
MeOH
Chapter 3 Solutions
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
Ch. 3.1 - In the following diagram, the white spheres...Ch. 3.1 - In the following digram, the white spheres...Ch. 3.1 - The unbalanced equation for the reaction between...Ch. 3.1 - Balance these equations by providing the missing...Ch. 3.2 - Which of the following reactions is the balanced...Ch. 3.2 - Write a balanced equation for (a) solid...Ch. 3.2 - Write the balanced equation for the reaction that...Ch. 3.2 - Prob. 3.4.2PECh. 3.3 - Which of the following is the correct formula...Ch. 3.3 - Prob. 3.5.2PE
Ch. 3.3 - What is the percentage of nitrogen, by mass, in...Ch. 3.3 - Calculate the percentage of potassium by mass in...Ch. 3.4 - Which of the following samples contains the fewest...Ch. 3.4 - Without using a calculator, arrange these samples...Ch. 3.4 -
How many sulfur are in (a) 0.45 mol BaSo4 and (b)...Ch. 3.4 -
How many oxygen atoms are in (a) 0.25 mol...Ch. 3.4 - A sample of an ionic compound containing iron and...Ch. 3.4 - Calculate the molar mass of Ca(NO3)2Ch. 3.4 - A 508-g sample of sodium bicarbonate (NaHCO3)...Ch. 3.4 - Prob. 3.10.2PECh. 3.4 - What is the mass, in grams, of 6.33 mol of NaHC03...Ch. 3.4 - What is the mass, in grams, of (a) 0.50 mol of...Ch. 3.4 - How many chlorine atoms are in 12.2 g of CCL4? a....Ch. 3.4 -
a. How many nitric acid molecules are in 4.20 g...Ch. 3.5 - A 2.144-g sample of phosgene, a compound used as a...Ch. 3.5 - A 5.325-g sample of methyl benzoate, a compound...Ch. 3.5 -
Cyclohexane a commonly used organic solvent, is...Ch. 3.5 - Prob. 3.14.2PECh. 3.5 -
The compound dioxane, which is used as a solvent...Ch. 3.5 -
a. Caproic acid, responsible for the odor of...Ch. 3.6 - Sodium hydroxide reacts with carbon dioxide to...Ch. 3.6 - Decomposition of KCIO3 is sometimes used to...Ch. 3.6 - Propane, C3 H8 (Figure 3.8), is a common fuel used...Ch. 3.6 -
Methanol, CH3OH, reacts with oxygen from air in a...Ch. 3.7 - When 24 mol of methanol and 15 mol of oxygen...Ch. 3.7 - a. When 1.50 mol of Al and 3.00 mol of Cl2 combine...Ch. 3.7 - Molten gallium reacts with arsenic to form the...Ch. 3.7 -
When a 2.00-g strip of zinc metal is placed in...Ch. 3.7 - If 3.00 g of titanium metal is reacted with 6.00 g...Ch. 3.7 - Imagine you are working on ways to improve the...Ch. 3 - The reaction between reactant A (blue spheres) and...Ch. 3 - The following diagram shows the combination...Ch. 3 -
3.3 The following diagram represents the...Ch. 3 -
3.4 The following diagram represents the...Ch. 3 - Glycine, an amino acid used by organisms to make...Ch. 3 - The following diagram represents a...Ch. 3 -
3.7 Nitrogen (N2) and hydrogen (H2) react to form...Ch. 3 -
3.8 Nitrogen monoxide and oxygen react to form...Ch. 3 - Write "true" or "false" for each statement. a. We...Ch. 3 - A key step in balancing chemical equations is...Ch. 3 - Balance the following equations: a. CO(g)...Ch. 3 - Balance the following equations 3. Li(s) + N2(g)...Ch. 3 -
3.13 Balance the following equations:
A14C3(s) +...Ch. 3 -
3.14 Balance the following equations:
a. Ca3P2(s)...Ch. 3 -
3.15 Write balanced chemical equations...Ch. 3 - Prob. 16ECh. 3 - Prob. 17ECh. 3 -
318
a. When a compound containing C, H, and O is...Ch. 3 - Write a balanced chemical equation for the...Ch. 3 -
3.20 Write a balanced chemical equation for the...Ch. 3 - Balance the following equations and indicate...Ch. 3 - Balance the following equations and indicate...Ch. 3 - Determine the formula weights of each of the...Ch. 3 - 3.24 Determine the formula weights of each of the...Ch. 3 - Calculate the percentage by mass of oxygen in the...Ch. 3 - Calculate the percentage by mass of the indicated...Ch. 3 - Based on the following structural formulas,...Ch. 3 - Calculate the percentage of carbon by mass In each...Ch. 3 - Write "true' or 'Yalse' for each statement a- A...Ch. 3 - a. What is the mass, in grams, of one mole of 12C?...Ch. 3 - Without doing any detailed calculations (but using...Ch. 3 - Without doing any detailed calculations {but using...Ch. 3 - What is the mass, m Iqlograms, of an Avogadro“s...Ch. 3 - If Avogadro’s number of pennies is divided equally...Ch. 3 - Calculate the following quantities: a. mass, in...Ch. 3 - Calculate the following quantities: a. mass, in...Ch. 3 - a. What is the mass, in grams, of 2.50 x 10-3 mol...Ch. 3 - a. What is the mass, in grams, of 1.223 mol of...Ch. 3 -
339 The molecular formula of allicin, the...Ch. 3 -
3.40 The molecular formula of aspartame, the...Ch. 3 -
3.41 A sample of glucose, C6H12O6, contains 1.250...Ch. 3 - A sample of the male sex hormone testosterone,...Ch. 3 -
343 The allowable concentration level of vinyl...Ch. 3 - At least 25 g oftetrahydrocannabinol (THC), the...Ch. 3 - Give the empirical formula of each of the...Ch. 3 - Determine the empirical formula of each of the...Ch. 3 - Determine the empirical formulas of the compounds...Ch. 3 - Determine the empirical formulas of the compounds...Ch. 3 -
3.49 A compound whose empirical formula is XF3...Ch. 3 - The compound XCL4contains 75.0% CI by mass What is...Ch. 3 -
3.51 What is the molecular formula of each of the...Ch. 3 - What is the molecular formula of each of the...Ch. 3 - Determine the empirical and molecular formulas of...Ch. 3 - Determine the empirical and molecular formulas of...Ch. 3 - a. Combustion analysis of toluene, a common...Ch. 3 - a. The characteristic odor of pineapple is due to...Ch. 3 -
3.57 Valproic acid, used to treat seizures and...Ch. 3 - Propenoic acid, C3H4O2, is a reactive organic...Ch. 3 -
3.59 Washing soda, a compound used to prepare...Ch. 3 -
3.60 Epsom salts, a strong laxative used in...Ch. 3 - f 3461 Hydrofluoric acid, HF(aq), cannot be stored...Ch. 3 - The reaction between potassium superoxide, KO2,...Ch. 3 -
3,63 Several brands of antacids use Al(OH)3 to...Ch. 3 -
3.64 An iron ore sample contains Fe2O3 together...Ch. 3 - Aluminum sulfides reacts with water to form...Ch. 3 - Calcium hydride reacts with water to form calcium...Ch. 3 -
3.67 Automotive air bags infilate when sodium...Ch. 3 - The complete combustion of octane, Cngs, a...Ch. 3 -
3.69 A piece of aluminum foil 1.00 cm2 and...Ch. 3 - Detonation of nitroglycerin proceeds as follows:...Ch. 3 -
3.71 The combustion of one mole of liquid...Ch. 3 - The combustion of one mole of liquid octane,...Ch. 3 - a. Define the terms limiting reactant and excess...Ch. 3 - Define the terms theoretical yield, actual yield,...Ch. 3 -
3-75 Consider the mixture of ethanol, C2H5OH, and...Ch. 3 - Consider the mixture of propane, C3H8, and O2...Ch. 3 -
3-77 Sodium hydroxide reacts with carbon doxidc...Ch. 3 -
3.78 Aluminum hydroxide reacts with sulfuric acid...Ch. 3 - The fizz produced when an Alka-Seltzer tablet is...Ch. 3 - One of the steps in the commercial procas for...Ch. 3 - Solutions of sodium carbonate and silver nitrate...Ch. 3 - Solutions of sulfuric acid and Iead (ll) acetate...Ch. 3 - When benzene (C6H6) reacts with bromine (Br2),...Ch. 3 - When ethane (C6H6) reacts with chlorine (Cl2), the...Ch. 3 -
3.85 Hydrogen sulfide is an impurity in natural...Ch. 3 -
386 When hydrogen sulfide gas is bubbled into a...Ch. 3 -
387 Write the balanced chemical equations for
a....Ch. 3 - If 1.5 mol C2H5OH, 1.5 mol C3H8, and 1.5 mol...Ch. 3 -
3.89 The effectiveness of nitrogen fertilizers...Ch. 3 -
3.90
a. The molecular formula of acetylsalicylic...Ch. 3 - Very small semiconductor crystals, composed of...Ch. 3 - a. One molecule of the antibiotic penicillin G has...Ch. 3 - Serotonin is a compound that conducts nerve...Ch. 3 -
3.94 The koala dines exclusively on eucalyptus...Ch. 3 -
3.95 Vanillin, the dominant flavoring in vanilla,...Ch. 3 -
3.96 An organic compound was found to contain...Ch. 3 -
3.97 A compound, KBrO,, where x is unknown, is...Ch. 3 - 398 An element X forms an iodide (X13) and a...Ch. 3 - A method used by the U.S. Environmental Protection...Ch. 3 -
3.100 A chemical plant uses electrical energy to...Ch. 3 - The fat stored in a camel’s hump is a source of...Ch. 3 -
3.102 When hydrocarbons are burned in a limited...Ch. 3 -
3.103 A mixture of N2(g) and H2(g) reacts in a...Ch. 3 -
3.104 A mixture containing KClO3, K2CO3, KHCO3,...Ch. 3 - When a mixture of 10.0 g of acetylene (C2H2) and...Ch. 3 -
3.106 The semiconductor gallium arsenide, GaAs,...Ch. 3 -
3.107 Paclitaxel, C47H51NO14, is an anticancer...Ch. 3 -
3.108 Consider a sample of calcium carbonate in...Ch. 3 -
3.109
a. You are given a cube of silver metal...Ch. 3 -
3.110
a. If an automobile travels 225 mi with a...Ch. 3 - Prob. 111IECh. 3 -
3.112 A particular coal contains 2.596 sulfur by...Ch. 3 - Hydrogen cyanide, HCN, is a poisonous gas. The...Ch. 3 -
3.114 The source of oxygen that drives he...Ch. 3 -
3.115 The therrnite reaction, Fe2O3 +Al Al2O3 +...Ch. 3 -
3.116 One of the most bizarre reactions in...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the correct IUPAC name for the compound shown here. Reset cis- 5- trans- ☑ 4-6- 2- 1- 3- di iso tert- tri cyclo sec- oct but hept prop hex pent yl yne ene anearrow_forwardQ6: Predict the major product(s) for the following reactions. Note the mechanism (SN1, SN2, E1 or E2) the reaction proceeds through. If no reaction takes place, indicate why. Pay attention to stereochemistry. NaCN DMF Br σ Ilm... Br H Br H H NaCN CH3OH KOtBu tBuOH NaBr H₂O LDA Et2O (CH3)2CHOH KCN DMSO NaOH H₂O, A LDA LDA Systemarrow_forwardQ7: For the following reactions, indicate the reaction conditions that would provide the indicated product in a high yield. Note the major reaction pathway that would take place (SN1, SN2, E1, or E2) Note: There may be other products that are not shown. There maybe more than one plausible pathway. Br H3C OH H3C CI ... H3C SCH2CH3 CI i SCH2CH3 ཨ་ Br System Settarrow_forward
- Q2: Rank the compounds in each of the following groups in order of decreasing rate of solvolysis in aqueous acetone. OSO2CF3 OSO2CH3 OH a. b. CI Brarrow_forwardох 4-tert-butyl oxy cyclohex-1-ene Incorrect, 1 attempt remaining The systematic name of this compound classifies the -OR group as a substituent of the hydrocarbon, which is considered the principal functional group. The ether substituent is named with the suffix 'oxy'. The general format for the systematic name of a hydrocarbon is: [prefix/substituent] + [parent] + [functional group suffix] Substituents are listed in alphabetical order. Molecules with a chiral center will indicate the absolute configuration at the beginning of its name with the R and S notation.arrow_forward5. Compressibility (6 points total). The isothermal compressibility is a measure of how hard/easy it is to compress an object (how squishy is it?) at constant temperature. It is др defined as Br=-()=-(200²)T' (a) You might wonder why there is a negative sign in this formula. What does it mean when this quantity is positive and what does it mean when this quantity is negative? (b) Derive the formula for the isothermal compressibility of an ideal gas (it is very simple!) (c) Explain under what conditions for the ideal gas the compressibility is higher or lower, and why that makes sense.arrow_forward
- 19. (3 pts) in Chapter 7 we will see a reaction of halocyclohexanes that requires that the halogen occupy an axial position with this in mind, would you expect cis-1-bromo-3-methylcyclohexane or trans-1-bromo-3-methylcyclohexane to be more reactive in this reaction? Briefly explain your choice using structures to support your answer. Mere-eries-cecleone) The tran-i-browse-3-methylcyclohexionearrow_forwardPlease help me calculate the undiluted samples ppm concentration. My calculations were 280.11 ppm. Please see if I did my math correctly using the following standard curve. Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EVSJL_W0qrxMkUjK2J3xMUEBHDu0UM1vPKQ-bc9HTcYXDQ?e=hVuPC4arrow_forwardProvide an IUPAC name for each of the compounds shown. (Specify (E)/(Z) stereochemistry, if relevant, for straight chain alkenes only. Pay attention to commas, dashes, etc.) H₁₂C C(CH3)3 C=C H3C CH3 CH3CH2CH CI CH3 Submit Answer Retry Entire Group 2 more group attempts remaining Previous Nextarrow_forward
- Arrange the following compounds / ions in increasing nucleophilicity (least to most nucleophilic) CH3NH2 CH3C=C: CH3COO 1 2 3 5 Multiple Choice 1 point 1, 2, 3 2, 1, 3 3, 1, 2 2, 3, 1 The other answers are not correct 0000arrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY