
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
9th Edition
ISBN: 9781305701021
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30.SE, Problem 14MP
Plastic photochromic sunglasses are based on the following reversible rearrangement of a dye inside the lenses that occurs when the lenses are exposed to sunlight. The original dye absorbs UV light but not visible light and is thus colorless, while the rearrangement product absorbs visible light and is thus darkened.
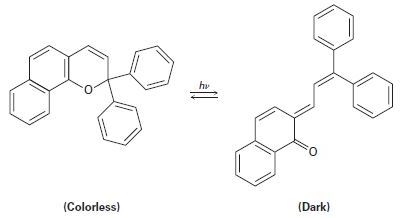
(a) Show the mechanism of the rearrangement.
(b) Why does the rearrangement product absorb at a longer wavelength (visible light) than the original dye (UV)?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?
Please predict the products for each of the
following reactions.
Clearly show the regiochemistry (Markovnikov
vs anti-Markovnikov) and stereochemistry
(syn- vs anti- or both).
If a mixture of enantiomers is formed, please
draw all the enantiomers.
Hint: In this case you must choose the best
answer to demonstrate the stereochemistry of
H2 addition.
1.03
2. (CH3)2S
BIZ
CH₂OH
2. DMS
KMnO4, NaOH
ΖΗ
Pd or Pt (catalyst)
HBr
20 1
HBr
ROOR (peroxide)
HO
H-SO
HC
12 11 10
BH, THE
2. H2O2, NaOH
Brz
cold
HI
19
18
17
16
MCPBA
15
14
13
A
Br
H₂O
BH3⚫THF
Brz
EtOH
Pd or Ni (catalyst)
D₂ (deuterium)
1. Os04
2. H2O2
CH3CO3H
(peroxyacid)
1. MCPBA
2. H₂O*
H
B
+
H
H
H
"H
C
H
H
D
Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.
Chapter 30 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 30.1 - Prob. 1PCh. 30.3 - Prob. 2PCh. 30.3 - Prob. 3PCh. 30.4 - Prob. 4PCh. 30.6 - What stereochemistry would you expect for the...Ch. 30.6 - Prob. 6PCh. 30.7 - Prob. 7PCh. 30.8 - Propose a mechanism to account for the fact that...Ch. 30.8 - When a 2, 6-disubstituted allyl phenyl ether is...Ch. 30.9 - Prob. 10P
Ch. 30.SE - Predict the product obtained when the following...Ch. 30.SE - Prob. 12VCCh. 30.SE - The following rearrangement of N-allyl-N,...Ch. 30.SE - Plastic photochromic sunglasses are based on the...Ch. 30.SE - Prob. 15MPCh. 30.SE - Prob. 16MPCh. 30.SE - Prob. 17MPCh. 30.SE - Prob. 18APCh. 30.SE - Prob. 19APCh. 30.SE - Prob. 20APCh. 30.SE - Prob. 21APCh. 30.SE - Prob. 22APCh. 30.SE - Prob. 23APCh. 30.SE - Prob. 24APCh. 30.SE - Prob. 25APCh. 30.SE - Prob. 26APCh. 30.SE - Prob. 27APCh. 30.SE - Prob. 28APCh. 30.SE - Propose a pericyclic mechanism to account for the...Ch. 30.SE - Prob. 30APCh. 30.SE - Prob. 31APCh. 30.SE - Prob. 32APCh. 30.SE - Prob. 33APCh. 30.SE - Bicyclohexadiene, also known as Dewar benzene, is...Ch. 30.SE - Prob. 35APCh. 30.SE - Prob. 36APCh. 30.SE - The 1H NMR spectrum of bullvalene at 100 C...Ch. 30.SE - Prob. 38APCh. 30.SE - Prob. 39APCh. 30.SE - Prob. 40APCh. 30.SE - In light of your answer to Problem 30-40, explain...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
- Using Benzene as starting materid show how each of the Following molecules Contel Ve syntheswed CHI 9. b -50311 с CHY 503H Ночто d. อ •NOV e 11-0-650 NO2arrow_forwardThe molecule PYRIDINE, 6th electrons and is therefore aromatre and is Assigned the Following structure contering Since aromatk moleculoy undergo electrophilic anomatic substitution, Pyridine shodd undergo The Following reaction + HNO3 12504 a. write all of the possible Mononitration Products that could Result From this reaction 18. Bared upon the reaction mechanison determime which of these producty would be the major Product of the hegetionarrow_forwarda. Explain Why electron withdrawing groups tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures fo. Explain why -ll is an outho -tura drccton even though chlorine has a very High Electronegativityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY