
COLLEGE PHYSICS
2nd Edition
ISBN: 9781711470832
Author: OpenStax
Publisher: XANEDU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30, Problem 30PE
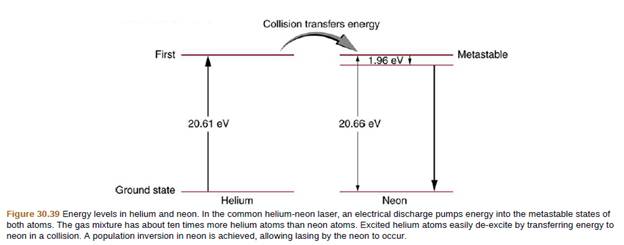
Figure 30.39 shows the energy-level diagram for neon. (a) Verity that the energy of the photon emitted when neon goes from its metastable state to the one immediately below is equal to 1.96 eV. (b) Show that the wavelength of this
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
You want to fabricate a soft microfluidic chip like the one below. How would you go about
fabricating this chip knowing that you are targeting a channel with a square cross-sectional
profile of 200 μm by 200 μm. What materials and steps would you use and why? Disregard the
process to form the inlet and outlet.
Square Cross Section
1. What are the key steps involved in the fabrication of a semiconductor device.
2. You are hired by a chip manufacturing company, and you are asked to prepare a silicon wafer
with the pattern below. Describe the process you would use.
High Aspect
Ratio
Trenches
Undoped Si Wafer
P-doped Si
3. You would like to deposit material within a high aspect ratio trench. What approach would you
use and why?
4. A person is setting up a small clean room space to carry out an outreach activity to educate high
school students about patterning using photolithography. They obtained a positive photoresist, a
used spin coater, a high energy light lamp for exposure and ordered a plastic transparency mask
with a pattern on it to reduce cost. Upon trying this set up multiple times they find that the full
resist gets developed, and they are unable to transfer the pattern onto the resist. Help them
troubleshoot and find out why pattern of transfer has not been successful.
5. You are given a composite…
Two complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values.
Any complex value can be expessed in the form of a+bi=reiθ. Find r and θ for (z1-z∗2)/z1+z2∗. Find r and θ for (z1−z2∗)z1z2∗ Please show all steps
Chapter 30 Solutions
COLLEGE PHYSICS
Ch. 30 - Name three different types of evidence for the...Ch. 30 - Explain why patterns observed in the periodic...Ch. 30 - If atoms exist, why can't we see them with visible...Ch. 30 - What two pieces of evidence allowed the first...Ch. 30 - How do the allowed orbits for electrons in atoms...Ch. 30 - How do the allowed orbits for electrons in atoms...Ch. 30 - Explain how Bohr's rule for the quantization of...Ch. 30 - What is a hydrogen-like atom, and how are the...Ch. 30 - Explain why characteristic x rays are the most...Ch. 30 - Why does the energy of characteristic x rays...
Ch. 30 - Observers at a safe distance from atmospheric test...Ch. 30 - Lasers are used to burn and read CDs. Explain why...Ch. 30 - Crystal lattices can be examined with x rays but...Ch. 30 - CT scanners do not detect details smaller than...Ch. 30 - How do the allowed orbits for electrons in atoms...Ch. 30 - Atomic and molecular spectra are discrete. What...Ch. 30 - Hydrogen gas can only absorb EM radiation that has...Ch. 30 - Lasers are used to burn and read CDs. Explain why...Ch. 30 - The coating on the inside of fluorescent light...Ch. 30 - What is the difference between fluorescence and...Ch. 30 - How can you tell that a hologram is a true...Ch. 30 - How is the de Broglie wavelength of electrons...Ch. 30 - What is the Zeeman effect, and what type of...Ch. 30 - Define the quantum numbers n,l,ml,s, and ms.Ch. 30 - For a given value of n, what are the allowed...Ch. 30 - For a given value of l, what are the allowed...Ch. 30 - List all the possible values of s and msfor an...Ch. 30 - Identify the shell, subshell, and number of...Ch. 30 - Which of the following are not allowed? State...Ch. 30 - Using the given charge-to-mass ratios for...Ch. 30 - (a) Calculate the mass of a proton using the...Ch. 30 - If someone wanted to build a scale model of the...Ch. 30 - Rutherford found the size of the nucleus to be...Ch. 30 - In Millikan's oil-drop experiment, one looks at a...Ch. 30 - (a) An aspiring physicist wants to build a scale...Ch. 30 - By calculating its wavelength, show that the first...Ch. 30 - Find the wavelength of the third line in the Lyman...Ch. 30 - Look up the values of the quantities in...Ch. 30 - Verify that the ground state energy E0 is 13.6 eV...Ch. 30 - If a hydrogen atom has its electron in the n=4...Ch. 30 - A hydrogen atom in an excited state can be ionized...Ch. 30 - Find the radius of a hydrogen atom in the n=2...Ch. 30 - Show that (13.6eV)/hc=1.097107m=R (Rydberg's...Ch. 30 - What is the smallest-wavelength line in the Balmer...Ch. 30 - Show that the entire Paschen series is in the...Ch. 30 - Do the Balmer and Lyman series overlap? To answer...Ch. 30 - (a) Which line in the Balmer series is the first...Ch. 30 - A wavelength of 4.653 m is observed in a hydrogen...Ch. 30 - A singly ionized helium ion has only one electron...Ch. 30 - A beryllium ion with a single electron (denoted...Ch. 30 - Atoms can be ionized by thermal collisions, such...Ch. 30 - Verify Equations rn=n2ZaB and...Ch. 30 - The wavelength of the four Balmer series lines for...Ch. 30 - (a) What is the shortest-wavelength x-ray...Ch. 30 - A color television tube also generates some x rays...Ch. 30 - An x ray tube has an applied voltage of 100 kV....Ch. 30 - The maximum characteristic x-ray photon energy...Ch. 30 - What are the approximate energies of the K and K...Ch. 30 - Figure 30.39 shows the energy-level diagram for...Ch. 30 - A helium-neon laser is pumped by electric...Ch. 30 - Ruby lasers have chromium atoms doped in an...Ch. 30 - (a) What energy photons can pump chromium atoms in...Ch. 30 - Some of the most powerful lasers are based on the...Ch. 30 - If an atom has an electron in the n=5 state with...Ch. 30 - An atom has an electron with m1=2. What is the...Ch. 30 - What are the possible values of m1 for an electron...Ch. 30 - What, if any, constraints does a value of ml=1...Ch. 30 - (a) Calculate the magnitude of the angular...Ch. 30 - (a) What is the magnitude of the angular momentum...Ch. 30 - Repeat Exercise 30.40 for l=3.Ch. 30 - (a) How many angles can L make with the z-axis for...Ch. 30 - What angles can the spin S of an electron make...Ch. 30 - (a) How many electrons can be in the n=4 shell?...Ch. 30 - (a) What is the minimum value of 1 for a subshell...Ch. 30 - (a) If one subshell of an atom has 9 electrons in...Ch. 30 - (a) List all possible sets of quantum numbers...Ch. 30 - Which of the following spectroscopic notations are...Ch. 30 - Which of the following spectroscopic notations are...Ch. 30 - (a) Using the Pauli exclusion principle and the...Ch. 30 - Integrated Concepts Estimate the density of a...Ch. 30 - Integrated Concepts The electric and magnetic...Ch. 30 - (a) What is the distance between the slits of a...Ch. 30 - Integrated Concepts A galaxy moving away from the...Ch. 30 - Integrated Concepts Calculate the velocity of a...Ch. 30 - Integrated Concepts In a Millikan oil-drop...Ch. 30 - Integrated Concepts What double-slit separation...Ch. 30 - Integrated Concepts In a laboratory experiment...Ch. 30 - Integrated Concepts Find the value of l, the...Ch. 30 - Integrated Concepts Particles called muons exist...Ch. 30 - Integrated Concepts Calculate the minimum amount...Ch. 30 - Integrated Concepts A carbon dioxide laser used in...Ch. 30 - Integrated Concepts Suppose an MRI scanner uses...Ch. 30 - Integrated Concepts (a) An excimer laser used for...Ch. 30 - Integrated Concepts A neighboring galaxy rotates...Ch. 30 - Integrated Concepts A pulsar is a rapidly spinning...Ch. 30 - Integrated Concepts Prove that the velocity of...Ch. 30 - Unreasonable Results (a) What voltage must be...Ch. 30 - Unreasonable Results A student in a physics...Ch. 30 - Construct Your Own Problem The solar corona is so...Ch. 30 - Construct Your Own Problem Consider the...Ch. 30 - Prob. 1TPCh. 30 - Prob. 2TPCh. 30 - Prob. 3TPCh. 30 - Prob. 4TPCh. 30 - Prob. 5TPCh. 30 - Prob. 6TPCh. 30 - Prob. 7TPCh. 30 - Prob. 8TPCh. 30 - Prob. 9TPCh. 30 - Prob. 10TPCh. 30 - Prob. 11TPCh. 30 - Prob. 12TPCh. 30 - Prob. 13TPCh. 30 - Prob. 14TPCh. 30 - Prob. 15TPCh. 30 - Prob. 16TP
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
1. An object is subject to two forces that do not point in opposite directions. Is it possible to choose their ...
College Physics: A Strategic Approach (3rd Edition)
16. A geneticist crosses a pure-breeding strain of peas producing yellow, wrinkled seeds with one that is pure...
Genetic Analysis: An Integrated Approach (3rd Edition)
For the generic equilibrium HA(aq) ⇌ H + (aq) + A- (aq), which of these statements is true?
The equilibrium con...
Chemistry: The Central Science (14th Edition)
17. Anthropologists are interested in locating areas in Africa where fossils 4-8 million years old might be fou...
Campbell Biology: Concepts & Connections (9th Edition)
7. Which bones form via intramembranous ossification?
a. Irregular bones
b. Certain flat bones
c. Long bones
d....
Human Anatomy & Physiology (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- An electromagnetic wave is traveling through vacuum in the positive x direction. Its electric field vector is given by E=E0sin(kx−ωt)j^,where j^ is the unit vector in the y direction. If B0 is the amplitude of the magnetic field vector, find the complete expression for the magnetic field vector B→ of the wave. What is the Poynting vector S(x,t), that is, the power per unit area associated with the electromagnetic wave described in the problem introduction? Give your answer in terms of some or all of the variables E0, B0, k, x, ω, t, and μ0. Specify the direction of the Poynting vector using the unit vectors i^, j^, and k^ as appropriate. Please explain all stepsarrow_forwardAnother worker is performing a task with an RWL of only 9 kg and is lifting 18 kg, giving him an LI of 2.0 (high risk). Questions:What is the primary issue according to NIOSH?Name two factors of the RWL that could be improved to reduce risk.If the horizontal distance is reduced from 50 cm to 30 cm, how does the HM change and what effect would it have?arrow_forwardTwo complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values. Any complex value can be expessed in the form of a+bi=reiθ. Find r and θ for z1z2∗. Find r and θ for z1/z2∗? Find r and θ for (z1−z2)∗/z1+z2∗. Find r and θ for (z1−z2)∗/z1z2∗ Please explain all steps, Thank youarrow_forward
- An ac series circuit consists of a voltage source of frequency 60 Hz and voltage amplitude V, a 505-Ω resistor, and a capacitor of capacitance 7.2 μF. What must be the source voltage amplitude V for the average electrical power consumed in the resistor to be 236 W? There is no inductance in the circuit.arrow_forwardAn L−R−C series circuit has R= 280 Ω . At the frequency of the source, the inductor has reactance XLL= 905 Ω and the capacitor has reactance XC= 485 Ω . The amplitude of the voltage across the inductor is 445 V . What is the amplitude of the voltage across the resistor and the capacitor? What is the voltage amplitude of the source? What is the rate at which the source is delivering electrical energy to the circuit?arrow_forwardA 0.185 H inductor is connected in series with a 98.5 Ω resistor and an ac source. The voltage across the inductor is vL=−(12.5V)sin[(476rad/s)t]vL. Derive an expression for the voltage vR across the resistor. Express your answer in terms of the variables L, R, VL (amplitude of the voltage across the inductor), ω, and t. What is vR at 2.13 ms ? Please explain all stepsarrow_forward
- A worker lifts a box under the following conditions:Horizontal distance (H): 30 cmInitial height (V): 60 cmVertical travel (D): 50 cmTorso rotation (A): 30°Frequency: 3 times/minute for 1 hourGrip: Good Question:What is the RWL for this task?What does this value mean in terms of occupational safety?arrow_forwardCan someone helparrow_forwardCan someone help mearrow_forward
- 3. Four identical small masses are connected in a flat perfect square. Rank the relative rotational inertias (IA, IB, IC) about the three axes of rotation shown. Axes A and B are in the plane of the square, and axis C is perpendicular to the plane, through mass m1. ΙΑ IB m2 m1 m3 Ic m4 (a) IAarrow_forwardConsider the circuit shown in the figure below. (Assume L = 5.20 m and R2 = 440 Ω.) (a) When the switch is in position a, for what value of R1 will the circuit have a time constant of 15.4 µs? (b) What is the current in the inductor at the instant the switch is thrown to position b?arrow_forwardCan someone helparrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax

Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning