
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30, Problem 30.58P
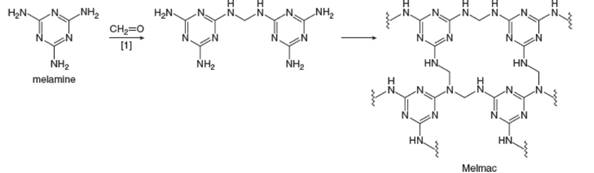
Melmac, a thermosetting
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a.
H3C.
N
H3C
CH3
HCN
ол
2.
восцапан
(46:00)
Curtius rearrangment
1. NaN3, heat
-OH
Question 1. Please predict the products for each of the following reactions.
Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both).
If a mixture of enantiomers is formed, please draw all the enantiomers.
Chapter 30 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 30 - Prob. 30.1PCh. 30 - Prob. 30.2PCh. 30 - Prob. 30.3PCh. 30 - Draw the mechanism for the radical polymerization...Ch. 30 - Prob. 30.5PCh. 30 - Prob. 30.6PCh. 30 - Prob. 30.7PCh. 30 - Prob. 30.8PCh. 30 - Prob. 30.9PCh. 30 - Prob. 30.10P
Ch. 30 - Prob. 30.11PCh. 30 - Problem 30.12
What polymer is formed by anionic...Ch. 30 - Prob. 30.13PCh. 30 - Prob. 30.14PCh. 30 - Problem 30.15
What polyamide is formed from each...Ch. 30 - Prob. 30.16PCh. 30 - Prob. 30.17PCh. 30 - Prob. 30.18PCh. 30 - Prob. 30.19PCh. 30 - Prob. 30.20PCh. 30 - Prob. 30.21PCh. 30 - Prob. 30.22PCh. 30 - Prob. 30.23PCh. 30 - Prob. 30.24PCh. 30 - Prob. 30.25PCh. 30 - 30.26 Draw the structure of the polymer formed by...Ch. 30 - Prob. 30.27PCh. 30 - Prob. 30.28PCh. 30 - Prob. 30.29PCh. 30 - 30.30 Draw each polymer in Problem 30.29 using the...Ch. 30 - Prob. 30.31PCh. 30 - Prob. 30.32PCh. 30 - Prob. 30.33PCh. 30 - Prob. 30.34PCh. 30 - Prob. 30.35PCh. 30 - Prob. 30.36PCh. 30 - Prob. 30.37PCh. 30 - Prob. 30.38PCh. 30 - 30.39 Draw a stepwise mechanism for the...Ch. 30 - 30.40 Cationic polymerization of 3-phenylpropene ...Ch. 30 - Prob. 30.41PCh. 30 - Prob. 30.42PCh. 30 - 30.43 Although styrene undergoes both cationic and...Ch. 30 - 30.44 Rank the following compounds in order of...Ch. 30 - Prob. 30.45PCh. 30 - Prob. 30.46PCh. 30 - 30.47 Draw a stepwise mechanism for the following...Ch. 30 - 30.48 Draw a stepwise mechanism for the reaction...Ch. 30 - 30.49 Draw the products of each reaction.
a. e....Ch. 30 - Prob. 30.50PCh. 30 - Prob. 30.51PCh. 30 - 30.52 (a) Explain why poly (vinyl alcohol) cannot...Ch. 30 - 30.53 Devise a synthesis of terephthalic acid and...Ch. 30 - Prob. 30.54PCh. 30 - Prob. 30.55PCh. 30 - 30.56 Compound A is a novel poly (ester amide)...Ch. 30 - 30.57 Researchers at Rutgers University have...Ch. 30 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 30 - 30.59 Although chain branching in radical...Ch. 30 - Prob. 30.60P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forwardExplain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forward
- Explain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forwardBriefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY