
Quinapril (trade name Accupril) is a drug used to treat hypertension and congestive heart
failure.
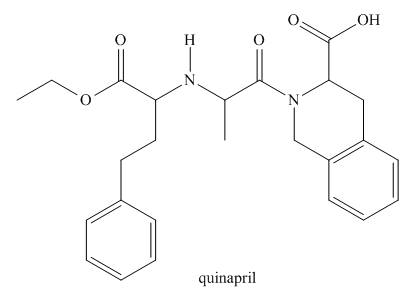
a. Identify the functional groups in quinpril.
b. Classify any alcohol, amide or
c. At which sites can quinapril hydrogen bond to water?
d. At which sites can quinapril hydrogen bond to acetone
e. Label the most acidic hydrogen atom.
f. Which site is most basic?
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
ORGANIC CHEMISTRY-ACCESS
- Reason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forwardIn electrochemistry, briefly describe the Galvani potential, the Volta potential, and the surface potential. Differentiate between them.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

