
Concept explainers
a)
Interpretation:
The group should be determined to which Z belongs in the following Lewis structure and along with an example of such a compound or ion which actually exists.
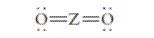
Concept Introduction:
Number of lone pair and bond pair electrons on an atom can give the number of valence electrons. This is indicated by the group number of an atom.
Group number is equal to the valence shell electrons of an atom or electrons present in the outermost shell. Valence shell electrons of the atom take part in the bonding.
Formal charge on an atom is defined as the charge assigned to the atom in a molecule. It is assigned to an atom or molecule by assuming that the electrons in
Formal charge is equal to
b)
Interpretation:
The group should be determined to which Z belongs in the following Lewis structure and along with an example of such a compound or ion which actually exists.
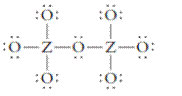
Concept Introduction:
Number of lone pair and bond pair electrons on an atom can give the number of valence electrons. This is indicated by the group number of an atom.
Group number is equal to the valence shell electrons of an atom or electrons present in the outermost shell. Valence shell electrons of the atom take part in the bonding.
Formal charge on an atom is defined as the charge assigned to the atom in a molecule. It is assigned to an atom or molecule by assuming that the electrons in chemical bonds are shared equally between atoms, apart from relative electronegativity.
Formal charge is equal to
c)
Interpretation:
The group should be determined to which Z belongs in the following Lewis structure and along with an example of such a compound or ion which actually exists.
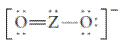
Concept Introduction:
Number of lone pair and bond pair electrons on an atom can give the number of valence electrons. This is indicated by the group number of an atom.
Group number is equal to the valence shell electrons of an atom or electrons present in the outermost shell. Valence shell electrons of the atom take part in the bonding.
Formal charge on an atom is defined as the charge assigned to the atom in a molecule. It is assigned to an atom or molecule by assuming that the electrons in chemical bonds are shared equally between atoms, apart from relative electronegativity.
Formal charge is equal to
d)
Interpretation:
The group should be determined to which Z belongs in the following Lewis structure and along with an example of such a compound or ion which actually exists.
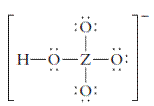
Concept Introduction:
Number of lone pair and bond pair electrons on an atom can give the number of valence electrons. This is indicated by the group number of an atom.
Group number is equal to the valence shell electrons of an atom or electrons present in the outermost shell. Valence shell electrons of the atom take part in the bonding.
Formal charge on an atom is defined as the charge assigned to the atom in a molecule. It is assigned to an atom or molecule by assuming that the electrons in chemical bonds are shared equally between atoms, apart from relative electronegativity.
Formal charge is equal to
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
PRINCIPLES OF MODERN CHEMISTRY
- Write all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





