
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
10th Edition
ISBN: 9781264116546
Author: Denniston
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 3.91QP
(a)
Interpretation Introduction
Interpretation:
The error in the given Lewis structure has to be found and the correct structures has to be written.
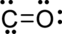
(b)
Interpretation Introduction
Interpretation:
The error in the given Lewis structure has to be found and the correct structures has to be written.
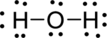
(c)
Interpretation Introduction
Interpretation:
The error in the given Lewis structure has to be found and the correct structures has to be written.
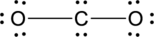
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If
two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each
reagent set. If a reaction cannot be carried out with reagents (sets)
class, write NP (not possible) in the solvent box for reagent set #1.
Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s).
Solvents: CH2Cl2 (A);
H₂O (B);
Reagents:
HBr (1);
H2SO4 (2);
CH3OH (C);
Br₂ (3);
CH3CO₂H (D)
NaHCO3 (4);
Hg(OAc)2 (5);
R₂BH (6);
H₂O₂ / HO- (7);
NaBH4 (8)
Reagent Set #1
Reagent Set #2
FGI
Solvent Reagent(s)
Solvent Reagent(s)
HO
OH
For which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeS
In the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two?
Group of answer choices
KCl(s) enthalpy of formation
Ionization energy for K(g)
K(s) sublimation enthalpy
Cl2 bond dissociation enthalpy
Chapter 3 Solutions
GENERAL,ORGANIC,+BIOCHEM.(LL) >CUSTOM<
Ch. 3.1 - Draw the Lewis symbol for oxygen, and indicate the...Ch. 3.1 - Prob. 3.2PPCh. 3.1 - Prob. 3.3PPCh. 3.2 - Prob. 3.4PPCh. 3.2 - Prob. 3.5PPCh. 3.2 - Prob. 3.1QCh. 3.2 - Prob. 3.2QCh. 3.2 - Prob. 3.6PPCh. 3.2 - Prob. 3.7PPCh. 3.2 - Prob. 3.8PP
Ch. 3.2 - Prob. 3.9PPCh. 3.4 - Prob. 3.10PPCh. 3.4 - Prob. 3.11PPCh. 3.4 - Prob. 3.12PPCh. 3.4 - Prob. 3.13PPCh. 3.4 - Prob. 3.3QCh. 3.4 - Prob. 3.4QCh. 3.4 - Prob. 3.14PPCh. 3.4 - Prob. 3.5QCh. 3.4 - Prob. 3.6QCh. 3.4 - Prob. 3.16PPCh. 3.4 - Prob. 3.7QCh. 3.4 - Prob. 3.8QCh. 3.4 - Prob. 3.9QCh. 3 - Prob. 3.13QPCh. 3 - Draw the appropriate Lewis symbol for each of the...Ch. 3 - Draw the appropriate Lewis symbol for each of the...Ch. 3 - Prob. 3.16QPCh. 3 - Describe the differences between covalent bonding...Ch. 3 - Describe the difference between nonpolar covalent...Ch. 3 - What is the periodic trend of electronegativity?
Ch. 3 - What role does electronegativity play in...Ch. 3 - Use electronegativity values to classify the bonds...Ch. 3 - Use electronegativity values to classify the bonds...Ch. 3 - When there is a reaction between each of these...Ch. 3 - Prob. 3.24QPCh. 3 - Explain, using Lewis symbols and the octet rule,...Ch. 3 - Explain, using Lewis symbols and the octet rule,...Ch. 3 - Prob. 3.27QPCh. 3 - Prob. 3.28QPCh. 3 - Name each of the following ions:
Na+
Cu+
Mg2+
Ch. 3 - Name each of the following ions:
Cu2+
Fe2+
Fe3+
Ch. 3 - Name each of the following ions:
HCO3–
H3O+
CO32−
Ch. 3 - Prob. 3.32QPCh. 3 - Prob. 3.33QPCh. 3 - Write the formula for each of the following...Ch. 3 - Prob. 3.35QPCh. 3 - Prob. 3.36QPCh. 3 - Prob. 3.37QPCh. 3 - Predict the formula of a compound formed...Ch. 3 - Prob. 3.39QPCh. 3 - Prob. 3.40QPCh. 3 - Prob. 3.41QPCh. 3 - Write the correct formula for each of the...Ch. 3 - Prob. 3.43QPCh. 3 - Write the correct formula for each of the...Ch. 3 - Prob. 3.45QPCh. 3 - Write the correct formula for each of the...Ch. 3 - Write a suitable formula for:
sodium...Ch. 3 - Write a suitable formula for:
aluminum...Ch. 3 - Prob. 3.49QPCh. 3 - Prob. 3.50QPCh. 3 - Prob. 3.51QPCh. 3 - Prob. 3.52QPCh. 3 - Write a suitable formula for:
silicon...Ch. 3 - Prob. 3.54QPCh. 3 - Contrast ionic and covalent compounds with respect...Ch. 3 - Prob. 3.56QPCh. 3 - Prob. 3.57QPCh. 3 - Prob. 3.58QPCh. 3 - Prob. 3.59QPCh. 3 - Prob. 3.60QPCh. 3 - Prob. 3.61QPCh. 3 - Prob. 3.62QPCh. 3 - Prob. 3.63QPCh. 3 - Prob. 3.64QPCh. 3 - Prob. 3.65QPCh. 3 - Prob. 3.66QPCh. 3 - How is the positive charge of a polyatomic cation...Ch. 3 - Prob. 3.68QPCh. 3 - Prob. 3.69QPCh. 3 - Prob. 3.70QPCh. 3 - Prob. 3.71QPCh. 3 - Prob. 3.72QPCh. 3 - Prob. 3.73QPCh. 3 - Prob. 3.74QPCh. 3 - Prob. 3.75QPCh. 3 - Prob. 3.76QPCh. 3 - Prob. 3.77QPCh. 3 - Prob. 3.78QPCh. 3 - Prob. 3.79QPCh. 3 - Prob. 3.80QPCh. 3 - Prob. 3.81QPCh. 3 - Prob. 3.82QPCh. 3 - Prob. 3.83QPCh. 3 - Prob. 3.84QPCh. 3 - Prob. 3.85QPCh. 3 - Prob. 3.86QPCh. 3 - Prob. 3.87QPCh. 3 - Prob. 3.88QPCh. 3 - Prob. 3.89QPCh. 3 - Prob. 3.90QPCh. 3 - Prob. 3.91QPCh. 3 - Prob. 3.93QPCh. 3 - Prob. 3.94QPCh. 3 - Prob. 3.95QPCh. 3 - Prob. 3.96QPCh. 3 - Prob. 3.97QPCh. 3 - Prob. 3.98QPCh. 3 - Prob. 3.99QPCh. 3 - Prob. 3.100QPCh. 3 - Prob. 3.101QPCh. 3 - Prob. 3.102QPCh. 3 - Prob. 3.103QPCh. 3 - Prob. 3.104QPCh. 3 - Prob. 3.107QPCh. 3 - Prob. 3.108QPCh. 3 - Prob. 3.109QPCh. 3 - Prob. 3.110QPCh. 3 - Prob. 3.112QPCh. 3 - Prob. 1MCPCh. 3 - Prob. 2MCPCh. 3 - Prob. 3MCPCh. 3 - Prob. 4MCPCh. 3 - Prob. 5MCPCh. 3 - Prob. 6MCPCh. 3 - Prob. 7MCPCh. 3 - Prob. 8MCPCh. 3 - Prob. 9MCPCh. 3 - Prob. 10MCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forwardWhat is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forwardPLEASE ANSWER THE QUESTION!!!arrow_forward
- 3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting material (SM) into the Target molecule. For each FGI in your proposed synthesis, specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O; ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI. FGI 1. Me Starting Material Source of all carbons in the Target molecule (can use multiple copies) Me Me Target molecule + enantiomerarrow_forwardcurved arrows are used to illustate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction mechanism stepsarrow_forwardIf is was a very hot day, what would the aldol condensation product be? *see imagearrow_forward
- Please help me with number 1-3. Thank you so much.arrow_forwardDraw the major product of this reaction ingnore the inorganic byproducts. 1. NaOCH2CH3 at 25 C 2. PhCH2Br (1 eq)arrow_forwardAt 90ºC the vapor pressure of ortho-xylene is 20 kPa and that of meta-xylene is 18 kPa. What is the composition of the vapor in equilibrium with a mixture in which the mole fraction of o-xylene is 0.60?arrow_forward
- Draw the products of this reduction of a ketone with sodium borohydride. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 1) NaBH4 2) HCI/H2O Select to Drawarrow_forwardWhy do you think people who live at high altitudes are advised to add salt to water when boiling food like pasta? What mole fraction of NaCl is needed to raise the boiling point of H2O by 3˚C? Does the amount of salt added to water (typically about one teaspoon to four quarts of water) substantially change the boiling point? (Kb (H2O) = 0.51˚C/molal.)arrow_forwardpls help asaparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY