
Concept explainers
(a)
Interpretation:
The hybridization of each non-hydrogen atom in Octocrylene is to be determined.
Concept introduction:
A hybrid atomic orbital is a cross between two or more pure AOs from the valence shell of a single atom. Typically
Answer to Problem 3.54P
Atoms shown in blue color are
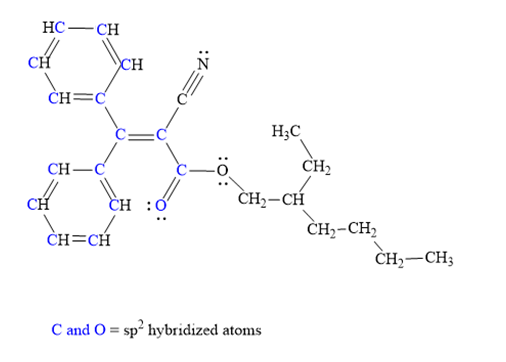
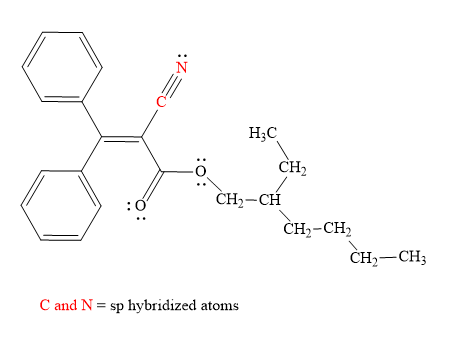
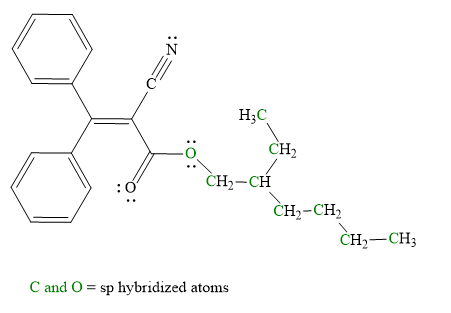
Explanation of Solution
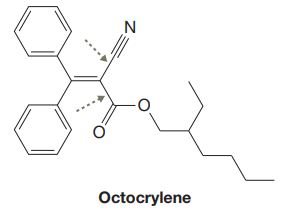
The given structure for Octocrylene is:
The above molecule has two benzene rings. Each carbon atom in the benzene ring has three electron groups: two single bonds and one double bond. Thus, the geometry for all these carbon atoms must be trigonal planar and hybridization must be

The triple bonded carbon atom has two electron groups: one single bond and one triple bond. Thus, the hybridization of this carbon atom must be

The single bonded oxygen atom and each carbon atom in the alkyl chain has four electrons groups. Thus, its geometry must be tetrahedral and hybridization must be

Hybridization of all non-hydrogen atoms is shown in three figures as above.
(b)
Interpretation:
All the atoms bonded to the acyclic
Concept introduction:
Free rotation can occur about single bonds but not about double bonds. According to the VSEPR theory, if an atom has three electron groups: three bonds, or two bonds and one pair of electrons then, the atom is said to be
Answer to Problem 3.54P
Explanation of Solution
The given structure for Octocrylene is:
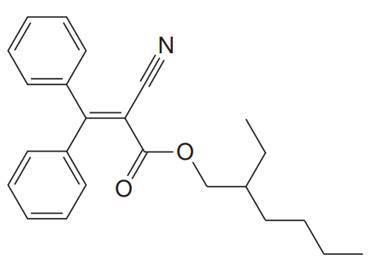
In the above structure, the
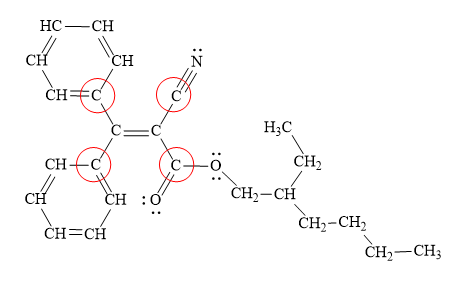
All the atoms bonded to the acyclic
(c)
Interpretation:
It is to be determined if there are two unique configurations possible about the acyclic
Concept introduction:
No free rotation takes place about the double bonds. Hence, in such compounds, molecules that differ by the exchange of two groups on one of the doubly bonded atoms are said to have different configurations. It is a cis configuration if the two non-hydrogen substituents are on present the same side of the double bond, and it is trans if they are on the opposite sides.
Answer to Problem 3.54P
It is not possible to have two unique configurations about the acyclic
Explanation of Solution
The given structure for Octocrylene is:
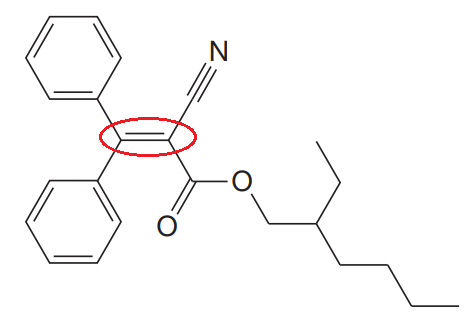
In the structure above, the acyclic
Thus, it is not possible to have two unique configurations about the acyclic
For a
(d)
Interpretation:
Out of the two indicated
Concept introduction:
The bond distance decrease and bond energies increase as the hybridization of the atom goes changes
Answer to Problem 3.54P
The
Explanation of Solution
The given structure for Octocrylene is:
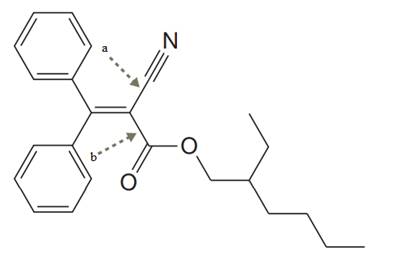
In the structure above, the two
The two carbon atoms involved in ‘a’ type of are
The two carbon atoms involved in ‘b’ type of bond are both
The bond distance decrease and bond energies increase as the hybridization of the atom goes changes
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry: Principles And Mechanisms
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

