
Write the expression for computing the pressure in a fluid.
Expression of pressure in a fluid
Explanation of Solution
Given:-
Density of fluid and depth of fluid.
In a fluid, if the fluid is stationary, the state of pressure at a point may be defined using pascal's law and hydrostatic law.
According to pascal's law, In a fluid, at a particular point, pressure acts uniformly and is equal in all the directions.
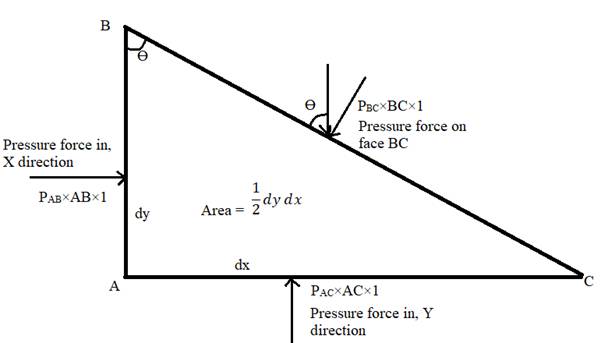
Resolving the forces in x direction,
Resolving the pressure forces in the y direction
Since the choice of fluid element is arbitrary, force on BC could be in any direction, Hence pressure at a point in all the direction is uniform and is equal in all direction.
The hydrostatic law states that the rate of increase with respect to depth in a stationary fluid is equal to the specific weight of the fluid.
To prove this, consider a small rectangular shaped fluid element of cross sectional area
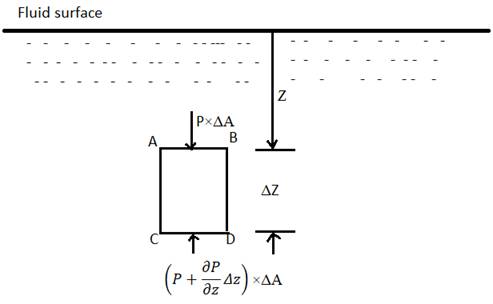
further at a given depth in a fluid, for a given density,
specific weight, w=
Hence to find the value of pressure at any depth Z, integrate both sides of eq2
Conclusion:
Pressure, P=
Want to see more full solutions like this?
Chapter 3 Solutions
EBK APPLIED FLUID MECHANICS
Additional Engineering Textbook Solutions
Electric Circuits. (11th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Database Concepts (8th Edition)
Concepts Of Programming Languages
Degarmo's Materials And Processes In Manufacturing
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
- Just do Questions 7, 9, 11. Here are notes attached for reference.arrow_forwardThis is a tilt and rotation question. Here are notes attached for reference.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A spring-loaded piston-cylinder device contains 1.5 kg of carbon dioxide. This system is heated from 200kPa and 25◦C to 1200 kPa and 300◦C. Determine the total heat transfer to and work produced by this system.arrow_forward
- Can you help with a code in MATLAB?arrow_forwardI need help writing a code in MATLAB. Please help me with question b.6arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes 1.5-kg of water that is initially at 90◦C with a quality of 5 percent occupies a spring-loaded piston-cylinder device. This device is now heated until the pressure rises to 900 kPa and the temperature is 280◦C. Determinethe total work produced during this process, in kJ.arrow_forward
- Thermodynamics: Mass and Energy Analysis Of Control Volumes Stainless steel ball bearings (ρ = 8085 kg/m3 and cp = 0.480 kJ/(kg◦C)) having a diameter of 1.5 cm areto be quenched in water at a rate of 900 per minute. The balls leave the oven at a uniform temperature of1000◦C and are exposed to air at 25◦C for a while before they are dropped into the water. If the temperatureof the balls drops to 900◦C prior to quenching, determine the rate of heat transfer from the balls to the air.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A 12-ft3 tank contains oxygen at 15 psia and 80◦F. A paddle wheel within the tank is rotated until thepressure inside rises to 20 psia. During the process 25 Btu of heat is lost to the surroundings. Determine thepaddle wheel work done. Neglect the energy stored in the paddle wheel.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A frictionless piston-cylinder device contains 4.5 kg of nitrogen at 110 kPa and 200 K. Nitrogen is nowcompressed slowly according to the relation PV1.5 = constant until it reaches a final temperature of 360 K.Calculate the work input during the process, in kJ.arrow_forward
- Thermodynamics: Mass and Energy Analysis Of Control Volumes An insulated piston-cylinder device contains 4 L of saturated liquid water at a constant pressure of 200 kPa.Water is stirred by a paddle wheel while a current of 8 A flows for 50 min through a resistor placed in thewater. If one-half of the liquid is evaporated during this constant-pressure process and the paddle-wheelwork amounts to 300 kJ, determine the voltage of the source. Also, show the process on a P–v diagram withrespect to the saturation lines.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes The state of liquid water is changed from 55 psia and 45◦F to 2000 psia and 120◦F. Determine the change inthe internal energy and enthalpy of water on the basis of the (a) compressed liquid tables, (b) incompressiblesubstance approximation and property tables, and (c) specific-heat model.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes What is the change in enthalpy, in kJ/kg, of oxygen as its temperature changes from 150 to 250◦C? Is thereany difference if the temperature change were from −50 to 100◦C? Does the pressure at the beginning andend of this process have any effect on the enthalpy change?arrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
