
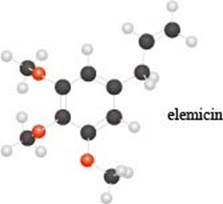
a. Identify the functional groups in the ball-and-stick model of elemicin, a compound partly responsible f or the flavor and fragrance of nutmeg.
b. Draw a skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point.
c. Label all electrophilic carbon atoms.
(a)
Interpretation: The functional groups in the ball-and-stick model of elemicin are to be identified.
Concept introduction: An atom or a group of atoms which are responsible for characteristic physical and chemical properties of the compound are collectively known as functional groups. The functional groups are the most reactive part present in the molecule.
Answer to Problem 31P
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
Explanation of Solution
The given molecule is,
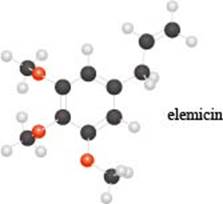
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of elemicin is,
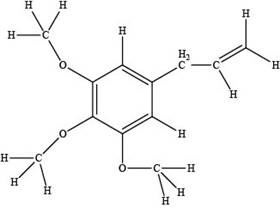
Figure 2
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
(b)
Interpretation: The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is to be drawn.
Concept introduction: The isomers which have same molecular formula but different connectivity of atoms are constitutional isomers.
The temperature at which the vapour pressure of a substance becomes equal to the pressure surrounding the liquid is boiling point. The boiling point depends on the intermolecular forces. Stronger the intermolecular force, higher is the boiling point.
The temperature at which the solid converts to its liquid phase is melting point. Stronger the intermolecular force, higher is the boiling point.
Answer to Problem 31P
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown below.
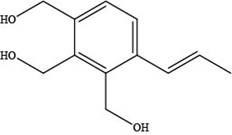
Explanation of Solution
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is,

Figure 3
This structure contains alcohol groups. This structure exhibits intermolecular hydrogen bonding due to the presence of hydrogen atoms bonded to oxygen atom. Hydrogen bonding is strongest intermolecular forces.
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown in Figure 3.
(c)
Interpretation: All electrophilic carbon atoms are to be labeled.
Concept introduction: An electron deficient due to hetero atoms or pi bonds or both is electrophilic site and an electron rich site due to hetero atoms or pi bonds or both is nucleophilic site.
Answer to Problem 31P
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Explanation of Solution
An oxygen atom is more electronegative than carbon atom which makes the carbon atom attached to oxygen atom electron deficient and electrophilic site as shown below.
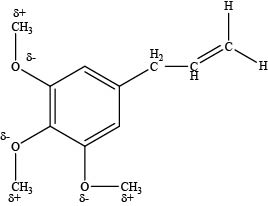
Figure 4
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry (6th Edition)
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward€ + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forward
- Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forward
- You may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forward
- Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forwardDraw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

