
The number of first-year, full-time enrollments expected in 2010 if the administrator used the data from 1975 to 1980 and to calculate the percentage error.
Answer to Problem 3.1EAA
The number of first-year full time enrollments expected in 2010 if the administrator used the data from 1975 to 1980 as 375000 students and the percentage error is 226%.
Explanation of Solution
Given information:
As per the given data, to find the error percentage upon usage of the data from 1975 to 1980 to predict the number of first-year full time enrollments expected in 2010.
According to the data in the Engineering for your future book, the following table illustrates the actual data for the years 1975 to 2010 and the predicted data for the year 2010 using only the estimates from 1975 to 1980.
| Year | Actual enrollments | Predicted enrollments |
| 1975 | 45000 | 45000 |
| 1980 | 118000 | 118000 |
| 1985 | 115000 | 143000 |
| 1990 | 90000 | 200000 |
| 1995 | 80000 | 237000 |
| 2000 | 82000 | 287000 |
| 2005 | 110000 | 328000 |
| 2010 | 115000 | 375000 |
The following is a graphical illustration of the same data in the table:
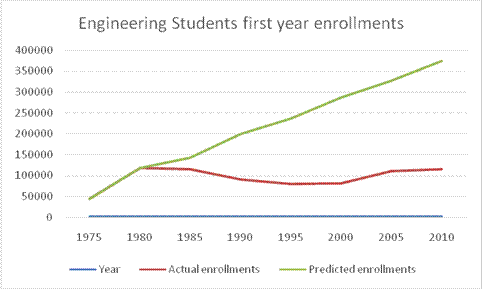
The above graph shows huge differences in the predicted data since it has taken the data belonging to the years 1975 to 1980 and prorated the same to the rest of the years until 2010. By doing this, the administrator has missed few important factors like the number of births every year; number of high school graduates every year, and other economic factors. These factors tend to change year after year and by taking only the data from 1975 to 1980, the number of enrollments for several years cannot be predicted accurately.
The percentage of error can be calculated as:
Percentage error
This shows that there is a huge percentage error upon the usage of the data from 1975 to 1980 to predict the number of first-year full time enrollments expected in 2010.
Conclusion:
Thus, the number of first year full-time enrollments expected in 2010 if the administrator used the data from 1975 to 1980 and the percentage error is discussed in details.
Want to see more full solutions like this?
- Thermodynamics: Mass and Energy Analysis Of Control Volumes 1.5-kg of water that is initially at 90◦C with a quality of 5 percent occupies a spring-loaded piston-cylinder device. This device is now heated until the pressure rises to 900 kPa and the temperature is 280◦C. Determinethe total work produced during this process, in kJ.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes Stainless steel ball bearings (ρ = 8085 kg/m3 and cp = 0.480 kJ/(kg◦C)) having a diameter of 1.5 cm areto be quenched in water at a rate of 900 per minute. The balls leave the oven at a uniform temperature of1000◦C and are exposed to air at 25◦C for a while before they are dropped into the water. If the temperatureof the balls drops to 900◦C prior to quenching, determine the rate of heat transfer from the balls to the air.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A 12-ft3 tank contains oxygen at 15 psia and 80◦F. A paddle wheel within the tank is rotated until thepressure inside rises to 20 psia. During the process 25 Btu of heat is lost to the surroundings. Determine thepaddle wheel work done. Neglect the energy stored in the paddle wheel.arrow_forward
- Thermodynamics: Mass and Energy Analysis Of Control Volumes A frictionless piston-cylinder device contains 4.5 kg of nitrogen at 110 kPa and 200 K. Nitrogen is nowcompressed slowly according to the relation PV1.5 = constant until it reaches a final temperature of 360 K.Calculate the work input during the process, in kJ.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes An insulated piston-cylinder device contains 4 L of saturated liquid water at a constant pressure of 200 kPa.Water is stirred by a paddle wheel while a current of 8 A flows for 50 min through a resistor placed in thewater. If one-half of the liquid is evaporated during this constant-pressure process and the paddle-wheelwork amounts to 300 kJ, determine the voltage of the source. Also, show the process on a P–v diagram withrespect to the saturation lines.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes The state of liquid water is changed from 55 psia and 45◦F to 2000 psia and 120◦F. Determine the change inthe internal energy and enthalpy of water on the basis of the (a) compressed liquid tables, (b) incompressiblesubstance approximation and property tables, and (c) specific-heat model.arrow_forward
- Thermodynamics: Mass and Energy Analysis Of Control Volumes What is the change in enthalpy, in kJ/kg, of oxygen as its temperature changes from 150 to 250◦C? Is thereany difference if the temperature change were from −50 to 100◦C? Does the pressure at the beginning andend of this process have any effect on the enthalpy change?arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A 50-L electrical radiator containing heating oil is placed in a 50-m3 room. Both the room and the oil in theradiator are initially at 5◦C. The radiator with a rating of 3 kW is now turned on. At the same time, heatis lost from the room at an average rate of 0.3 kJ/s. After some time, the average temperature is measuredto be 20◦C for the air in the room, and 60◦C for the oil in the radiator. Taking the density and the specificheat of the oil to be 950 kg/m3 and 2.2 kJ/(kg◦C), respectively, determine how long the heater is kept on.Assume the room is well-sealed so that there are no air leaks.arrow_forwardProblem 3 For the beam and loading shown, consider section n-n and determine (a) the largest shearing stress in that section, (b) the shearing stress at point a. 1ft 15 kips 20 kips 15 kips AITT in 1 0.6 in. -10 in. 1 in. 0.375 in.- 2 ft 2ft 2 ft 2ft 10 in. 1 0.6 in.arrow_forward
- practice problems want detailed break downarrow_forward6.105. Determine force P on the cable if the spring is compressed 0.025 m when the mechanism is in the position shown. The spring has a stiffness of k = 6 kN/m. E P 150 mm D T 30° 200 mm 200 mm 200 mm B 800 mmarrow_forward6.71. Determine the reactions at the supports A, C, and E of the compound beam. 3 kN/m 12 kN A B CD E -3 m 4 m 6 m 3 m 2 marrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





