
Concept explainers
Which compound in each pair has the higher boiling point?
a. 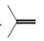 or
or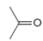 c.
c. 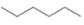 or
or
b. 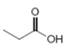 or
or 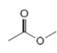 d.
d. 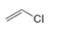 or
or 
(a)
Interpretation: In the given pair, the compound with the higher boiling point is to be predicted.
Concept introduction: Boiling point is defined as the temperature at which the compound is converted to a gaseous state.
The boiling point of compounds depends upon the molecular weight and the intermolecular forces present in the compounds. The intermolecular forces are the forces that exist in between the two or more atoms in a compound. Due to strong intermolecular forces, the molecule will require more energy to break the bonds. The compounds that have ionic bonding and hydrogen bonding will possess higher boiling point.
Answer to Problem 3.13P
In the given pair of compounds,
Explanation of Solution
The given compounds are
In
The increasing order of boiling point with intermolecular forces is,
Hence, the boiling point of
In the given pair of compounds,
(b)
Interpretation: In the given pair, the compound with the higher boiling point is to be predicted.
Concept introduction: Boiling point is defined as the temperature at which the compound is converted to a gaseous state.
The boiling point of compounds depends upon the molecular weight and the intermolecular forces present in the compounds. The intermolecular forces are the forces that exist in between the two or more atoms in a compound. Due to strong intermolecular forces, the molecule will require more energy to break the bonds. The compounds that have ionic bonding and hydrogen bonding will possess higher boiling point.
Answer to Problem 3.13P
In the given pair of compounds,
Explanation of Solution
The given compounds are
In
In the given pair of compounds,
(c)
Interpretation: In the given pair, the compound with the higher boiling point is to be predicted.
Concept introduction: Boiling point is defined as the temperature at which the compound is converted to a gaseous state.
The boiling point of compounds depends upon the molecular weight and the intermolecular forces present in the compounds. The intermolecular forces are the forces that exist in between the two or more atoms in a compound. Due to strong intermolecular forces, the molecule will require more energy to break the bonds. The compounds that have ionic bonding and hydrogen bonding will possess higher boiling point.
Answer to Problem 3.13P
In the given pair of compounds,
Explanation of Solution
The given compounds are
In both the compounds,
In the given pair of compounds,
(d)
Interpretation: In the given pair, the compound with the higher boiling point is to be predicted.
Concept introduction: Boiling point is defined as the temperature at which the compound is converted to a gaseous state.
The boiling point of compounds depends upon the molecular weight and the intermolecular forces present in the compounds. The intermolecular forces are the forces that exist in between the two or more atoms in a compound. Due to strong intermolecular forces, the molecule will require more energy to break the bonds. The compounds that have ionic bonding and hydrogen bonding will possess higher boiling point.
Answer to Problem 3.13P
In the given pair of compounds,
Explanation of Solution
The given compounds are
Van der Waals forces exist in both the compounds due to the presence of
In the given pair of compounds,
Want to see more full solutions like this?
Chapter 3 Solutions
PKG ORGANIC CHEMISTRY
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forward
- Predict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

