
To review:
The types of forces, which help the insulin to bind to its target, and the truth about the amino acids placed at positions B23, B24, A2, A19, and A3, which allow them to get involved in the substrate binding.
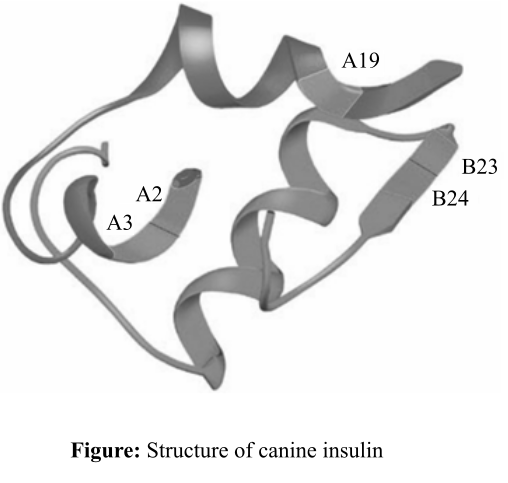
Figure: Structure of canine insulin.
Introduction:
The quaternary structure of the proteins involves two or more chains of the peptides that held together with the help of various covalent and noncovalent forces like hydrogen bonds, van der Waal forces of attraction, and disulfide linkages. An enzyme is held to its substrate with the help of van der Waal forces of attraction.
Explanation of Solution
Insulin is an enzyme, which helps to lower the blood glucose level by storing it in the form of glycogen in the muscle tissues. It binds to its substrate, that is, glucose by the noncovalent van der Waal interactions. The van der Waal interactions occur over a short distance and are weak forces. Generally, enzymes bind to their substrate with the help of these forces so that products formed could be detached easily from the enzyme and it again becomes available for the next cycle of reaction.
The part of the insulin enzyme, that is involved in van der Waal interaction, contains hydrophobic amino acid groups (−R group), for example, valine, isoleucine, glycine, and phenylalanine, which might have placed on the positions such as A2, A3, A19, B23, and B24. The –R groups protrude out of the insulin side chain so that they could interact with the target molecules or the amino acids that are present in the substrates.
Thus, it can be concluded that the noncovalent interactions like van der Waal forces help the insulin to bind to its substrates. Amino acids at the positions B23, B24, A2, A19, and A3 might contain hydrophobic side chain amino acid groups so that they help insulin to bind to substrates.
Want to see more full solutions like this?
- Describe the principle of homeostasis.arrow_forwardExplain how the hormones of the glands listed below travel around the body to target organs and tissues : Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forwardWhat are the functions of the hormones produced in the glands listed below: Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forward
- Describe the hormones produced in the glands listed below: Pituitary gland Hypothalamus Thyroid Parathyroid Adrenal Pineal Pancreas(islets of langerhans) Gonads (testes and ovaries) Placentaarrow_forwardPlease help me calculate drug dosage from the following information: Patient weight: 35 pounds, so 15.9 kilograms (got this by dividing 35 pounds by 2.2 kilograms) Drug dose: 0.05mg/kg Drug concentration: 2mg/mLarrow_forwardA 25-year-old woman presents to the emergency department with a 2-day history of fever, chills, severe headache, and confusion. She recently returned from a trip to sub-Saharan Africa, where she did not take malaria prophylaxis. On examination, she is febrile (39.8°C/103.6°F) and hypotensive. Laboratory studies reveal hemoglobin of 8.0 g/dL, platelet count of 50,000/μL, and evidence of hemoglobinuria. A peripheral blood smear shows ring forms and banana-shaped gametocytes. Which of the following Plasmodium species is most likely responsible for her severe symptoms? A. Plasmodium vivax B. Plasmodium ovale C. Plasmodium malariae D. Plasmodium falciparumarrow_forward
- please fill in missing parts , thank youarrow_forwardplease draw in the answers, thank youarrow_forwarda. On this first grid, assume that the DNA and RNA templates are read left to right. DNA DNA mRNA codon tRNA anticodon polypeptide _strand strand C с A T G A U G C A TRP b. Now do this AGAIN assuming that the DNA and RNA templates are read right to left. DNA DNA strand strand C mRNA codon tRNA anticodon polypeptide 0 A T G A U G с A TRParrow_forward
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning





