
Using Figure 3.1, determine
a. the number of grams of
b. the number of grams of water required to dissolve
c. the number of grams of water required to dissolve
d. the number of grams of water required to dissolve a mixture containing
(a)
Interpretation:
The number of grams of
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of
Explanation of Solution
The given illustration of the graph is shown below.
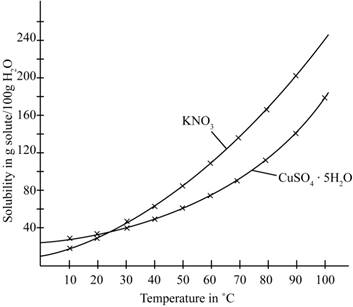
Figure 1
According to the above graph, the number of grams of
The number of grams of
(b)
Interpretation:
The number of grams of water required to dissolve
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve
Explanation of Solution
The given illustration of the graph is shown below.
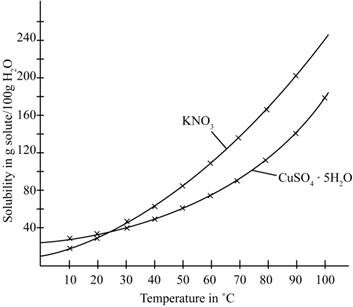
Figure 1
According to the above graph, the number of grams of
Thus, water required to dissolve
The number of grams of water required to dissolve
(c)
Interpretation:
The number of grams of water required to dissolve
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve
Explanation of Solution
The given illustration of the graph is shown below.
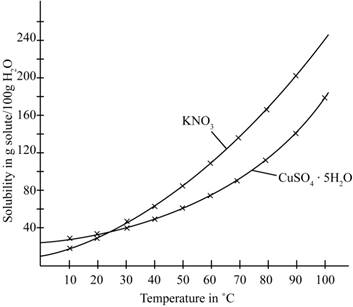
Figure 1
According to the above graph, the number of grams of
Thus, the water required to dissolve
The number of grams of water required to dissolve
(d)
Interpretation:
The number of grams of water required to dissolve a mixture containing
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve a mixture containing
Explanation of Solution
The given illustration of the graph is shown below.
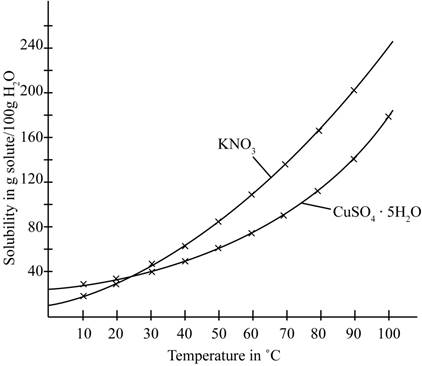
Figure 1
The amount of water required to dissolve
The amount of water required to dissolve
Therefore, the total amount of water required to dissolve a mixture containing
The number of grams of water required to dissolve a mixture containing
Want to see more full solutions like this?
Chapter 3 Solutions
Chemical Principles in the Laboratory
- Draw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forwardDraw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward
- 19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forwardIndicate the product of the reaction OH OH CH3-CC- Ph + H2SO4 a 20°C | CH3 Pharrow_forward
- 35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forwardpls helparrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





