
The reason that the electrons in the Rutherford model fall into the nucleus.
Answer to Problem 1RQ
Solution:
The reason for electrons to fall into the nucleus, according to the Rutherford atomic model, is the force of attraction between the positive nucleus and the negatively-charged electron.
Explanation of Solution
Introduction:
The Rutherford atomic model is also known as the planetary or nuclear model of an atom. This model designated the atom as a positively charged, dense, tiny core called as a nucleus. Electrons (negative constitutent of atom) constitute the mass of the atom. These electrons revolve at a certain distance from the nucleus similar to the way planets revolve around the Sun.
Explanation:
According to Rutherford’s postulate, electrons revolve at a very high speed around the nucleus of an atom in a particular orbit. However, accelerated charged particles release a certain amount of
The electron spirals inward towards the nucleus as shown in the diagram:
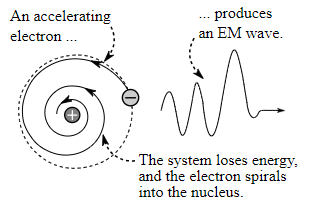
The given figure shows the emission of electromagnetic radiation by the accelerating electron as well as the shrinkage of the orbit of the electron.
Conclusion:
The force of attraction between the positive nucleus and the negatively-charged electron is the main cause for electrons to fall into the nucleus, according to the Rutherford atomic model.
Want to see more full solutions like this?
Chapter 28 Solutions
Modified Mastering Physics with Pearson eText -- Access Card -- for College Physics: Explore and Apply (18-Weeks)
- A rock is dropped from a height of 2.00 m. Determine the velocity of the rock just before it hits the ground. If the momentum of the rock just before hitting the ground is 14.0 kg m/s, what is the mass of the rock? Is the collision between the rock and the ground elastic or inelastic? Explain.arrow_forwardDescribe how the momentum of a single ball changes as it free falls from a height of approximately 1 m, collides with a hard floor, and rebounds.arrow_forward• Nature of Resistance Temperature-Resistance Relationship Ohm's Law, Energy and Power Kirchhoff's Law • • Maxwell's Mesh Analysis 1. The steel of the third rail of a railway system has a resistivity of 21.4 μ-cm. If its cross-sectional area is 8.2 in², calculate the resistance per mile of rail, neglecting the effect of joints between sections. (1 point) 2. An incandescent lamp has a tungsten filament whose resistance is 96 at its operating temperature of 2900°C. Calculate the filament resistance when the lamp is disconnected from the electric source, under which condition its temperature is 24°C. (Use do = 0.0045 02/°C for tungsten) (1 point) 3. For the circuit shown, find the following: 50 V 602 10 V 702 a. the value of resistor R. (1 point) b. the equivalent resistance with respect to the 50-V source. (1 point) 4. For the circuit shown, determine all the currents in each branch using Kirchhoff's Laws. (3 points) A 5V 2 В -ний C 4 6 VT ww F E 5. Use Maxwell's Mesh to find I, and VAB…arrow_forward
- For items 8-9, refer to the problem below. Find all the currents flowing in every resistor, power dissipation in every resistor and the total power of the circuit shown at the right using... 8. Kirchhoff's Laws (5 pts) 9. Maxwell's Mesh Analysis (5 pts) A 8 V 10 V B + 20 Ω 3Ω 202 wwww C wwww 202 + 50 www 12 Varrow_forward• Nature of Resistance Temperature-Resistance Relationship Ohm's Law, Energy and Power Kirchhoff's Law • Maxwell's Mesh Analysis 1. A coil of copper wire (p = 10.37 2-cmil/ft) has a length of 600 ft. What is the length of an aluminum conductor (p 17 cmil/ft), if its cross-sectional area and resistance are the same as those of the copper coil? (Hint: Look for conversion of inches to mils and square inches to square foot. Include it in your solution.) (1 pt) 2. The copper field winding of an electric machine has a resistance of 46 at temperature of 22°C. What will be its resistance at 75°C? (Use do = 0.00427 /°C for copper) (1 pt) 3. The resistivity of a copper rod 50 ft long and 0.25 inch in diameter is 1.76 μ at 20°C. What is its resistance at - 20°C? (1 pt) 4. When two resistors A and B are connected in series, the total resistance is 36 2. When connected in parallel, the total resistance is 8 Q. What is the ratio of the resistance RA to resistance RB? Assume RA < RB. (1 pt) 5. The…arrow_forward2. Two equally strong individuals, wearing exactly the same shoes decide to do a tug of war. The only difference is individual A is 2.5 meters tall and individual B is 1.5 meter tall. Who is more likely to win the tug of war?arrow_forward
- 6. A car drives at steady speed around a perfectly circular track. (a) The car's acceleration is zero. (b) The net force on the car is zero. (c) Both the acceleration and net force on the car point outward. (d) Both the acceleration and net force on the car point inward. (e) If there is no friction, the acceleration is outward.arrow_forward9. A spring has a force constant of 100 N/m and an unstretched length of 0.07 m. One end is attached to a post that is free to rotate in the center of a smooth. table, as shown in the top view in the figure below. The other end is attached to a 1kg disc moving in uniform circular motion on the table, which stretches the spring by 0.03 m. Friction is negligible. What is the centripetal force on the disc? Top View (a) 0.3 N (b) 3.0 N (c) 10 N (d) 300 N (e) 1000 Narrow_forward4. A child has a ball on the end of a cord, and whirls the ball in a vertical circle. Assuming the speed of the ball is constant (an approximation), when would the tension in the cord be greatest? (a) At the top of the circle. (b) At the bottom of the circle. (c) A little after the bottom of the circle when the ball is climbing. (d) A little before the bottom of the circle when the ball is descending quickly. (e) Nowhere; the cord is pulled the same amount at all points.arrow_forward
- 3. In a rotating vertical cylinder (Rotor-ride) a rider finds herself pressed with her back to the rotating wall. Which is the correct free-body diagram for her? (a) (b) (c) (d) (e)arrow_forward8. A roller coaster rounds the bottom of a circular loop at a nearly constant speed. At this point the net force on the coaster cart is (a) zero. (b) directed upward. (c) directed downward. (d) Cannot tell without knowing the exact speed.arrow_forward5. While driving fast around a sharp right turn, you find yourself pressing against the left car door. What is happening? (a) Centrifugal force is pushing you into the door. (b) The door is exerting a rightward force on you. (c) Both of the above. (d) Neither of the above.arrow_forward
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Horizons: Exploring the Universe (MindTap Course ...PhysicsISBN:9781305960961Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Horizons: Exploring the Universe (MindTap Course ...PhysicsISBN:9781305960961Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning





