
Physics for Scientists and Engineers with Modern Physics
10th Edition
ISBN: 9781337553292
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.7, Problem 2.7QQ
In Figure 2.12, match each vx–t graph on the top with the ax–t graph on the bottom that best describes the motion.
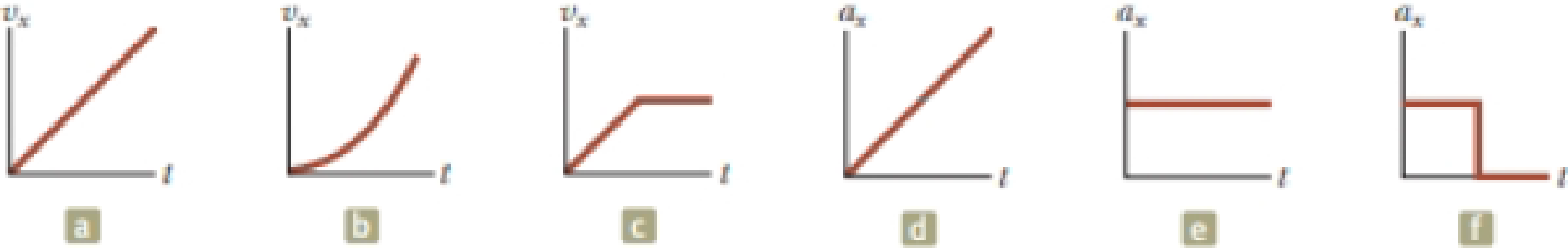
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2
3
Imagine you are out for a stroll on a sunny day when you encounter a lake. Unpolarized light from the sun is reflected off the lake into your eyes. However, you notice when you put on your vertically polarized sunglasses, the light reflected off the lake no longer reaches your eyes. What is the angle between the unpolarized light and the surface of the water, in degrees, measured from the horizontal? You may assume the index of refraction of air is nair=1 and the index of refraction of water is nwater=1.33 . Round your answer to three significant figures. Just enter the number, nothing else.
Chapter 2 Solutions
Physics for Scientists and Engineers with Modern Physics
Ch. 2.1 - Which of the following choices best describes what...Ch. 2.1 - Under which of the following conditions is the...Ch. 2.2 - Are officers in the highway patrol more interested...Ch. 2.5 - Make a velocitytime graph for the car in Figure...Ch. 2.5 - If a car is traveling eastward and slowing down,...Ch. 2.6 - Which one of the following statements is true? (a)...Ch. 2.7 - In Figure 2.12, match each vxt graph on the top...Ch. 2.8 - Consider the following choices: (a) increases, (b)...Ch. 2 - The speed of a nerve impulse in the human body is...Ch. 2 - A particle moves according to the equation x =...
Ch. 2 - The position of a pinewood derby car was observed...Ch. 2 - An athlete leaves one end of a pool of length L at...Ch. 2 - A positiontime graph for a particle moving along...Ch. 2 - A car travels along a straight line at a constant...Ch. 2 - A person takes a trip, driving with a constant...Ch. 2 - A child rolls a marble on a bent track that is 100...Ch. 2 - Figure P2.9 shows a graph of vx versus t for the...Ch. 2 - (a) Use the data in Problem 3 to construct a...Ch. 2 - A particle starts from rest and accelerates as...Ch. 2 - Draw motion diagrams for (a) an object moving to...Ch. 2 - Each of the strobe photographs (a), (b), and (c)...Ch. 2 - An electron in a cathode-ray tube accelerates...Ch. 2 - A parcel of air moving in a straight tube with a...Ch. 2 - In Example 2.7, we investigated a jet landing on...Ch. 2 - An object moving with uniform acceleration has a...Ch. 2 - Solve Example 2.8 by a graphical method. On the...Ch. 2 - A glider of length moves through a stationary...Ch. 2 - Why is the following situation impossible?...Ch. 2 - A glider of length 12.4 cm moves on an air track...Ch. 2 - In the particle under constant acceleration model,...Ch. 2 - At t = 0, one toy car is set rolling on a straight...Ch. 2 - You are observing the poles along the side of the...Ch. 2 - Prob. 25PCh. 2 - An attacker at the base of a castle wall 3.65 m...Ch. 2 - The height of a helicopter above the ground is...Ch. 2 - Prob. 28PCh. 2 - Prob. 29PCh. 2 - At time t = 0, a student throws a set of keys...Ch. 2 - Prob. 31PCh. 2 - A student drives a moped along a straight road as...Ch. 2 - Automotive engineers refer to the time rate of...Ch. 2 - In Figure 2.11b, the area under the velocitytime...Ch. 2 - The froghopper Philaenus spumarius is supposedly...Ch. 2 - A woman is reported to have fallen 144 ft from the...Ch. 2 - At t = 0, one athlete in a race running on a long,...Ch. 2 - Prob. 38APCh. 2 - Hannah tests her new sports car by racing with...Ch. 2 - Two objects, A and B, are connected by hinges to a...Ch. 2 - Prob. 41APCh. 2 - Two thin rods are fastened to the inside of a...Ch. 2 - In a womens 100-m race, accelerating uniformly,...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?arrow_forwardpls help on allarrow_forwardpls help on thesearrow_forward
- pls help on all asked questions kindlyarrow_forwardpls help on all asked questions kindlyarrow_forward19. Mount Everest, Earth's highest mountain above sea level, has a peak of 8849 m above sea level. Assume that sea level defines the height of Earth's surface. (re = 6.38 × 106 m, ME = 5.98 × 1024 kg, G = 6.67 × 10 -11 Nm²/kg²) a. Calculate the strength of Earth's gravitational field at a point at the peak of Mount Everest. b. What is the ratio of the strength of Earth's gravitational field at a point 644416m below the surface of the Earth to a point at the top of Mount Everest? C. A tourist watching the sunrise on top of Mount Everest observes a satellite orbiting Earth at an altitude 3580 km above his position. Determine the speed of the satellite.arrow_forward
- pls help on allarrow_forwardpls help on allarrow_forward6. As the distance between two charges decreases, the magnitude of the electric potential energy of the two-charge system: a) Always increases b) Always decreases c) Increases if the charges have the same sign, decreases if they have the opposite signs d) Increases if the charges have the opposite sign, decreases if they have the same sign 7. To analyze the motion of an elastic collision between two charged particles we use conservation of & a) Energy, Velocity b) Momentum, Force c) Mass, Momentum d) Energy, Momentum e) Kinetic Energy, Potential Energyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning

Classical Dynamics of Particles and Systems
Physics
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Stars and Galaxies
Physics
ISBN:9781305120785
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning
Half life | Radioactivity | Physics | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=IDkNlU7zKYU;License: Standard YouTube License, CC-BY