
(a)
Interpretation:
Chair conformations and the position of substituents need to be identified for the given set of compounds.
Concept introduction:
Ring-flipping is a phenomenon known as ring inversion that involves rotation about the single bonds of cyclic conformers. Usually this happens in cyclohexane ring. When ring flip happens in the cyclohexane, the axially and equatorially substituted groups are inverted. The ring-flipping also happens in a fused ring system. Decalin is a fused ring system. There are two stereoisomers for decalin, namely cis-decalin and trans-decalin. If the substituent is facing away from the ring or which makes
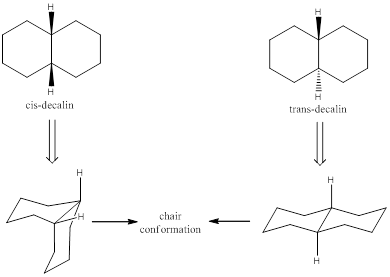
When viewing the molecule from the top the bonds which are said to be in parallel with the viewing angle are in axial position and which are not parallel are known to be in equatorial position.
To draw and explain: chair conformation and identify the substituent position.
(b)
Interpretation:
Chair conformations and the position of substituents need to be identified for the given set of compounds.
Concept introduction:
Ring-flipping is a phenomenon known as ring inversion that involves rotation about the single bonds of cyclic conformers. Usually this happens in cyclohexane ring. When ring flip happens in the cyclohexane, the axially and equatorially substituted groups are inverted. The ring-flipping also happens in a fused ring system. Decalin is a fused ring system. There are two stereoisomers for decalin, namely cis-decalin and trans-decalin. If the substituent is facing away from the ring or which makes

When viewing the molecule from the top the bonds which are said to be in parallel with the viewing angle are in axial position and which are not parallel are known to be in equatorial position.
To draw and explain: chair conformation and identify the substituent position.
(c)
Interpretation:
Chair conformations and the position of substituents need to be identified for the given set of compounds.
Concept introduction:
Ring-flipping is a phenomenon known as ring inversion that involves rotation about the single bonds of cyclic conformers. Usually this happens in cyclohexane ring. When ring flip happens in the cyclohexane, the axially and equatorially substituted groups are inverted. The ring-flipping also happens in a fused ring system. Decalin is a fused ring system. There are two stereoisomers for decalin, namely cis-decalin and trans-decalin. If the substituent is facing away from the ring or which makes

When viewing the molecule from the top the bonds which are said to be in parallel with the viewing angle are in axial position and which are not parallel are known to be in equatorial position.
To draw and explain: chair conformation and identify the substituent position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
ORGANIC CHEMISTRY-PRINT (LL)-W/WILEY
- Firefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forward
- Please draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forward
- CHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forwardTRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forward
- Calculate the pH of 0.0025 M phenol.arrow_forwardIn the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forwardUsing spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





