
(a)
Interpretation:
Structure of lecithin and the naturally occurring enantiomer need to be drawn and if phosphodiester is in C2 position will there be chirality has to be discussed.
Concept introduction:
Phosphoglycerides are same as triglycerides but the only difference is that in triglycerides there are three fatty acid residues. But in phosphoglyceride one of the fatty acid residue in triglyceride is replaced by a phosphoester. Hydrolysis of phosphoglycerides yield glycerol, fatty acid, phosphate.
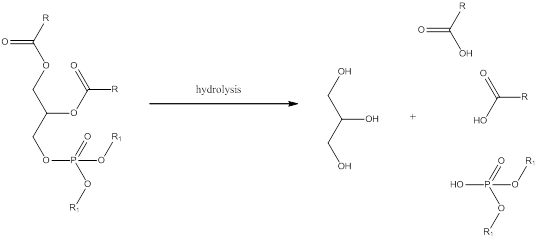
To draw: the structure of lecithin
(b)
Interpretation:
Structure of lecithin and the naturally occurring enantiomer need to be drawn and if phosphodiester is in C2 position will there be chirality has to be discussed.
Concept Introduction:
Chirality is the ability of the compound to rotate the plane polarized light when it is passed through them. A chiral center is the one when the carbon atom is attached to a four different groups. If the plane polarized light is rotated clockwise then the compound is said to be dextrorotatory and if it is anti-clockwise then the compound is levorotatory.
(c)
Interpretation:
Structure of lecithin and the naturally occurring enantiomer need to be drawn and if phosphodiester is in C2 position will there be chirality has to be discussed.
Concept Introduction:
Chirality is the ability of the compound to rotate the plane polarized light when it is passed through them. A chiral center is the one when the carbon atom is attached to a four different groups. If the plane polarized light is rotated clockwise then the compound is said to be dextrorotatory and if it is anti-clockwise then the compound is levorotatory. If the carbon is not attached to four different groups means then it is said to be achiral.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
ORGANIC CHEMISTRY (LL) >CUSTOM PACKAGE<
- Draw the Zaitsev product of the dehydration of this alcohol. + I X 5 OH ざ~ TSOH Click and drag to start drawing a structure.arrow_forwardPlease help with identifying these.arrow_forwardFor the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forward
- Can I please get this answered? With the correct number of significant digits.arrow_forwardDraw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forwardCan I please get help with this graph, if you could show exactly where it needs to pass through please.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





