
ORGANIC CHEMISTRY (LL) W/ACCESS
4th Edition
ISBN: 9781119856122
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 26.4, Problem 3LTS
Interpretation Introduction
Interpretation:
Mechanism for transesterification of trilaurin using ethanol in presence of acid catalyst need to be drawn.
Concept introduction:
Transesterification is a process in which the alcohol group present in the carboxylate of triglyceride is exchanged by another organic alcohol group. The general scheme can be shown as,
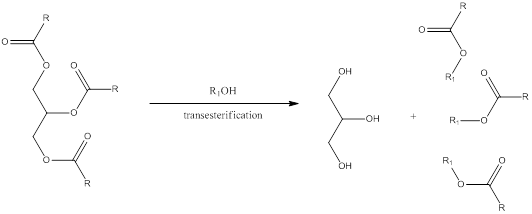
The steps involved in transesterification is,
- Protonation
- Nucleophilic attack
- Deprotonation
- Protonation
- Loss of leaving group
- Deprotonation
To draw: the mechanism of transesterification of trilaurin using ethanol in presence of acid catalyst.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
LIOT
S
How would you make 200. mL of a 0.5 M solution of CuSO4 5H2O from solid copper (II) sulfate?
View Rubric
Steps and explantions please
Match the denticity to the ligand.
Water
monodentate
✓
C₂O2
bidentate
H₂NCH₂NHCH2NH2 bidentate
x
EDTA
hexadentate
Question 12
Partially correct
Mark 2 out of 2
Flag question
Provide the required information for the coordination compound shown below:
Na NC-Ag-CN]
Number of ligands:
20
Coordination number: 2✔
Geometry: linear
Oxidation state of transition metal ion: +3 x
in 12
correct
out of 2
question
Provide the required information for the coordination compound shown below.
Na NC-Ag-CN]
Number of ligands:
20
Coordination number: 2
Geometry: linear
0
Oxidation state of transition metal ion:
+3X
Chapter 26 Solutions
ORGANIC CHEMISTRY (LL) W/ACCESS
Ch. 26.2 - Prob. 1CCCh. 26.2 - Prob. 2CCCh. 26.3 - Prob. 1LTSCh. 26.3 - Prob. 3PTSCh. 26.4 - Prob. 7CCCh. 26.4 - Prob. 8CCCh. 26.4 - Prob. 2LTSCh. 26.4 - Prob. 9PTSCh. 26.4 - Prob. 3LTSCh. 26.4 - Prob. 12PTS
Ch. 26.5 - Prob. 15CCCh. 26.5 - Prob. 16CCCh. 26.5 - Prob. 17CCCh. 26.5 - Prob. 18CCCh. 26.5 - Prob. 19CCCh. 26.6 - Prob. 20CCCh. 26.6 - Prob. 21CCCh. 26.6 - Prob. 22CCCh. 26.6 - Prob. 23CCCh. 26.7 - Prob. 24CCCh. 26.8 - Prob. 4LTSCh. 26.8 - Prob. 25PTSCh. 26.8 - Prob. 27CCCh. 26.8 - Prob. 28CCCh. 26 - Prob. 29PPCh. 26 - Prob. 30PPCh. 26 - Prob. 32PPCh. 26 - Prob. 33PPCh. 26 - Prob. 34PPCh. 26 - Prob. 35PPCh. 26 - Prob. 36PPCh. 26 - Prob. 37PPCh. 26 - Prob. 38PPCh. 26 - Prob. 39PPCh. 26 - Prob. 40PPCh. 26 - Prob. 41PPCh. 26 - Prob. 42PPCh. 26 - Prob. 43PPCh. 26 - Prob. 44PPCh. 26 - Prob. 45PPCh. 26 - Prob. 46PPCh. 26 - Prob. 47PPCh. 26 - Prob. 48PPCh. 26 - Prob. 49PPCh. 26 - Prob. 50PP
Knowledge Booster
Similar questions
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY