
ORGANIC CHEM. - ACCESS PKG+MODELING KIT
4th Edition
ISBN: 9781119838746
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 26.4, Problem 3LTS
Interpretation Introduction
Interpretation:
Mechanism for transesterification of trilaurin using ethanol in presence of acid catalyst need to be drawn.
Concept introduction:
Transesterification is a process in which the alcohol group present in the carboxylate of triglyceride is exchanged by another organic alcohol group. The general scheme can be shown as,
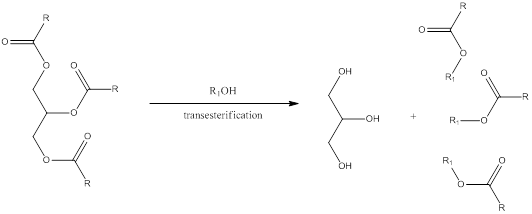
The steps involved in transesterification is,
- Protonation
- Nucleophilic attack
- Deprotonation
- Protonation
- Loss of leaving group
- Deprotonation
To draw: the mechanism of transesterification of trilaurin using ethanol in presence of acid catalyst.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Design experiments in UV-Vis to figure the optimal mole ratio of copper (1:1, 2:1, 3:1 and etc)versus ethambutol using all necessary chemicals including dihydrochloride and copper nitrate hemipentahydrate and sodium hydroxide. Show how UV-Vis absorbance and maximum wavelength would change in response
Correct each molecule in the drawing area below so that it has the condensed structure it would have if it were dissolv
a 0.1 M aqueous solution of HCI.
If there are no changes to be made, check the No changes box under the drawing area.
No changes.
HO—CH,—C—CH,—OH
X
5
2
2
2
HO–CH,—CH,—C—CH,—OH
Explanation
Check
Center Accessi
©2025 on
5
C
Make the calculations to prepare 2M H2SO4, from concentrated H2SO4 (98%; density: 1.84 g/mL).
Chapter 26 Solutions
ORGANIC CHEM. - ACCESS PKG+MODELING KIT
Ch. 26.2 - Prob. 1CCCh. 26.2 - Prob. 2CCCh. 26.3 - Prob. 1LTSCh. 26.3 - Prob. 3PTSCh. 26.4 - Prob. 7CCCh. 26.4 - Prob. 8CCCh. 26.4 - Prob. 2LTSCh. 26.4 - Prob. 9PTSCh. 26.4 - Prob. 3LTSCh. 26.4 - Prob. 12PTS
Ch. 26.5 - Prob. 15CCCh. 26.5 - Prob. 16CCCh. 26.5 - Prob. 17CCCh. 26.5 - Prob. 18CCCh. 26.5 - Prob. 19CCCh. 26.6 - Prob. 20CCCh. 26.6 - Prob. 21CCCh. 26.6 - Prob. 22CCCh. 26.6 - Prob. 23CCCh. 26.7 - Prob. 24CCCh. 26.8 - Prob. 4LTSCh. 26.8 - Prob. 25PTSCh. 26.8 - Prob. 27CCCh. 26.8 - Prob. 28CCCh. 26 - Prob. 29PPCh. 26 - Prob. 30PPCh. 26 - Prob. 32PPCh. 26 - Prob. 33PPCh. 26 - Prob. 34PPCh. 26 - Prob. 35PPCh. 26 - Prob. 36PPCh. 26 - Prob. 37PPCh. 26 - Prob. 38PPCh. 26 - Prob. 39PPCh. 26 - Prob. 40PPCh. 26 - Prob. 41PPCh. 26 - Prob. 42PPCh. 26 - Prob. 43PPCh. 26 - Prob. 44PPCh. 26 - Prob. 45PPCh. 26 - Prob. 46PPCh. 26 - Prob. 47PPCh. 26 - Prob. 48PPCh. 26 - Prob. 49PPCh. 26 - Prob. 50PP
Knowledge Booster
Similar questions
- H CH3 CH3 b) Write the products of your compound and the following reagents. If the reaction would not work for your compound, write "no reaction" and explain the problem. NaCN H* H₂NNHCH5 H* -à NaBH -à CH2MgBr Cro₁₂ --à H3O+ -à c) Would your compound give a positive Tollen's test? Why or why not?arrow_forwardHomework 4 Chem 204 Dr. Hellwig Consider this compound, which will be referred to as "your compound". a) Name your compound according to the IUPAC system. Include stereochemistry (E/Z/R/S) H CH3 CH3arrow_forwardWhat is the mechanism for this?arrow_forward
- 21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to synthesize the following ethers through Williamson ether synthesis. (a) (c) (d) (e) (f) H₂COarrow_forward1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward
- 4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forwardIn the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward
- 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forwarddraw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forwardDraw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY