
ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260826791
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 26, Problem 61P
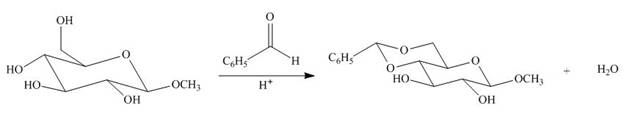
Draw a stepwise mechanism for the following reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the total energy cost associated with the compound below adopting the shown conformation?
CH3
HH
DH
CH3
ΗΝ,
Draw Final Product
C
cyclohexanone
pH 4-5
Edit Enamine
H3O+
CH3CH2Br
THF, reflux
H
Edit Iminium Ion
How many hydrogen atoms are connected to the indicated carbon atom?
Chapter 26 Solutions
ORGANIC CHEMISTRY
Ch. 26.2 - Prob. 1PCh. 26.2 - Prob. 2PCh. 26.2 - Label each stereogenic center as R or S. a. b. c....Ch. 26.2 - Convert the ball-and-stick model to a Fischer...Ch. 26.2 - Prob. 5PCh. 26.2 - Prob. 6PCh. 26.3 - Prob. 7PCh. 26.3 - Prob. 8PCh. 26.4 - Prob. 9PCh. 26.4 - Prob. 10P
Ch. 26.6 - Prob. 11PCh. 26.6 - Prob. 12PCh. 26.6 - Prob. 13PCh. 26.6 - Prob. 14PCh. 26.6 - Prob. 15PCh. 26.7 - Prob. 16PCh. 26.7 - Draw a stepwise mechanism for the following...Ch. 26.7 - Prob. 18PCh. 26.8 - Prob. 19PCh. 26.9 - Prob. 20PCh. 26.9 - Prob. 21PCh. 26.9 - Draw the products formed when D-arabinose is...Ch. 26.9 - Prob. 23PCh. 26.10 - Prob. 24PCh. 26.10 - Prob. 25PCh. 26.10 - Prob. 26PCh. 26.10 - Prob. 27PCh. 26.11 - Prob. 28PCh. 26.11 - Prob. 29PCh. 26.12 - Prob. 30PCh. 26.12 - Prob. 31PCh. 26.13 - Prob. 32PCh. 26.13 - Prob. 33PCh. 26.13 - Problem-28.35
Draw the structures of the...Ch. 26.13 - Prob. 35PCh. 26 - 28.37 Convert each ball-and-stick model to a...Ch. 26 - Prob. 37PCh. 26 - Prob. 38PCh. 26 - 28.40 Convert each compound to a Fischer...Ch. 26 - Prob. 40PCh. 26 - Prob. 41PCh. 26 - 28.43 Draw a Haworth projection for each compound...Ch. 26 - Prob. 43PCh. 26 - 28.45 Draw both pyranose anomers of each...Ch. 26 - Prob. 45PCh. 26 - 28.50 Draw the products formed when D-altrose is...Ch. 26 - 28.58 Draw a stepwise mechanism for the following...Ch. 26 - Prob. 62PCh. 26 - Prob. 63PCh. 26 - Prob. 64PCh. 26 - Prob. 65PCh. 26 - Prob. 66PCh. 26 - Prob. 67PCh. 26 - Prob. 68PCh. 26 - Prob. 69PCh. 26 - Prob. 70P
Additional Science Textbook Solutions
Find more solutions based on key concepts
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
What were the major microbiological interests of Martinus Beijerinck and Sergei Winogradsky? It can be said tha...
Brock Biology of Microorganisms (15th Edition)
2. Which of the following is the best example of the use of a referent? _
a. A red bicycle
b. Big as a dump tru...
Physical Science
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
11. In the early 1800s, French naturalist Jean Baptiste Lamarck suggested that the best explanation for the rel...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY