
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
9th Edition
ISBN: 9781305084391
Author: McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25.SE, Problem 33MP
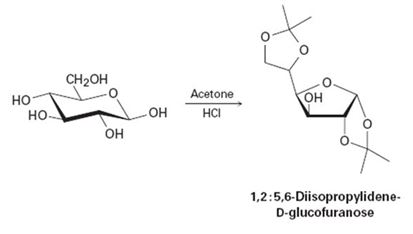
D-Glicose reacts with acetone in the presence of acid to yield the nonreducing 1, 2: 5, 6-diisopropylidene-D-glucofuranose. Propose a mechanism.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.
Chapter 25 Solutions
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
Ch. 25.1 - Prob. 1PCh. 25.2 - Prob. 2PCh. 25.2 - Prob. 3PCh. 25.2 - Prob. 4PCh. 25.2 - Prob. 5PCh. 25.3 - Prob. 6PCh. 25.3 - Prob. 7PCh. 25.4 - Prob. 8PCh. 25.4 - Prob. 9PCh. 25.4 - Prob. 10P
Ch. 25.5 - Prob. 11PCh. 25.5 - Prob. 12PCh. 25.5 - Prob. 13PCh. 25.5 - Prob. 14PCh. 25.5 - Prob. 15PCh. 25.6 - Prob. 16PCh. 25.6 - Prob. 17PCh. 25.6 - Prob. 18PCh. 25.6 - Prob. 19PCh. 25.6 - Prob. 20PCh. 25.6 - Prob. 21PCh. 25.6 - Prob. 22PCh. 25.6 - Prob. 23PCh. 25.7 - Prob. 24PCh. 25.8 - Show the product you would obtain from the...Ch. 25.SE - Prob. 26VCCh. 25.SE - Prob. 27VCCh. 25.SE - Prob. 28VCCh. 25.SE - Prob. 29VCCh. 25.SE - Prob. 30MPCh. 25.SE - Prob. 31MPCh. 25.SE - Glucosamine, one of the eight essential...Ch. 25.SE - D-Glicose reacts with acetone in the presence of...Ch. 25.SE - Prob. 34MPCh. 25.SE - Prob. 35MPCh. 25.SE - Prob. 36APCh. 25.SE - Prob. 37APCh. 25.SE - Prob. 38APCh. 25.SE - Prob. 39APCh. 25.SE - Prob. 40APCh. 25.SE - Assign R or S configuration to each chirality...Ch. 25.SE - Prob. 42APCh. 25.SE - Prob. 43APCh. 25.SE - Prob. 44APCh. 25.SE - Prob. 45APCh. 25.SE - Prob. 46APCh. 25.SE - Prob. 47APCh. 25.SE - Prob. 48APCh. 25.SE - Prob. 49APCh. 25.SE - Prob. 50APCh. 25.SE - Prob. 51APCh. 25.SE - Prob. 52APCh. 25.SE - Prob. 53APCh. 25.SE - Prob. 54APCh. 25.SE - Prob. 55APCh. 25.SE - Prob. 56APCh. 25.SE - Prob. 57APCh. 25.SE - Prob. 58APCh. 25.SE - Prob. 59APCh. 25.SE - Prob. 60APCh. 25.SE - Prob. 61APCh. 25.SE - Prob. 62APCh. 25.SE - Prob. 63APCh. 25.SE - D-Mannose reacts with acetone to give a...Ch. 25.SE - Prob. 65APCh. 25.SE - Prob. 66APCh. 25.SE - Prob. 67APCh. 25.SE - Prob. 68APCh. 25.SE - Prob. 69APCh. 25.SE - Prob. 70APCh. 25.SE - Prob. 71AP
Additional Science Textbook Solutions
Find more solutions based on key concepts
An aluminum calorimeter with a mass of 100 g contains 250 g of water. The calorimeter and water are in thermal ...
Physics for Scientists and Engineers
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Give the IUPAC name for each compound.
Organic Chemistry
6. How can you use the features found in each chapter?
Human Anatomy & Physiology (2nd Edition)
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY