
(a)
Interpretation:
The
Concept introduction:
Asymmetric catalytic hydrogenation is a reaction in which the alkene substrate is reduced to a saturated compound by addition of two atoms of hydrogen. When chiral catalyst is used a enantioselective product is obtained. This technique uses a chiral catalyst so that the amino acids can be prepared with high enantiomeric excess.
The final step after hydrogenation is the hydrolysis of the protected group of
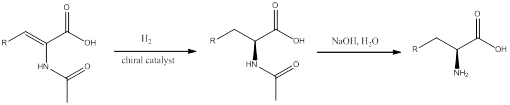
To find: the alkene taken for the synthesis of L-alanine using asymmetric catalytic hydrogenation.
(b)
Interpretation:
The alkenes required for the synthesis of given set of amino acids by asymmetric catalytic hydrogenation need to be identified.
Concept introduction:
Asymmetric catalytic hydrogenation is a reaction in which the alkene substrate is reduced to a saturated compound by addition of two atoms of hydrogen. When chiral catalyst is used a enantioselective product is obtained. This technique uses a chiral catalyst so that the amino acids can be prepared with high enantiomeric excess.
The final step after hydrogenation is the hydrolysis of the protected group of amine. The general scheme can be shown as,

To find: the alkene taken for the synthesis of L-alanine using asymmetric catalytic hydrogenation.
(c)
Interpretation:
The alkenes required for the synthesis of given set of amino acids by asymmetric catalytic hydrogenation need to be identified.
Concept introduction:
Asymmetric catalytic hydrogenation is a reaction in which the alkene substrate is reduced to a saturated compound by addition of two atoms of hydrogen. When chiral catalyst is used a enantioselective product is obtained. This technique uses a chiral catalyst so that the amino acids can be prepared with high enantiomeric excess.
The final step after hydrogenation is the hydrolysis of the protected group of amine. The general scheme can be shown as,

To find: the alkene taken for the synthesis of L-tyrosine using asymmetric catalytic hydrogenation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
Organic Chemistry
- Can I please get this answered? With the correct number of significant digits.arrow_forwardDraw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forwardCan I please get help with this graph, if you could show exactly where it needs to pass through please.arrow_forward
- Draw the condensed structure of 1,3-dihydroxy-2-pentanone. Explanation Check Click anywhere to draw the first atom of your structure. Х C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of use +arrow_forward0.500 moles of NOCl are placed into a 1.00 L vessesl at 700K and after the system comes to equilibrium, the consentration of NOCl is 0.440 M. Calculate the equilibrium constant Kc for the reaction: 2NOCL (g) --> 2NO (g) + Cl2 (g)arrow_forwardWhat is the hydronium ion concentration in a solution of water that has a hydroxide ion concentrationof 1.0 x 10-2 M?arrow_forward
- Identify conjugate acid-base pairs in the following reactions:HBr (aq) + H2O (l) ⇌ H3O+ (aq) + Br- (aq) - OH (aq) + CH3COOH (aq) ⇌ H2O (l) + CH3COO- (aq)arrow_forward4:45 PM Tue Apr 1 K 77% Problem 9 of 10 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting structure, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw any missing organic intermediates or products for this reaction. Include all lone pairs in the structures. Ignore inorganic byproducts, counterions, and solvents. :0: H Select to Add Arrows HI CH3OH H+ ·HO CH3OH, H+ 0:0 H H Select to Add Arrows tion Versirate CH3OH, H* Select to Draw Productarrow_forwardCan I please get help with this graph? If you can show exactly where it needs to pass through.arrow_forward
- G 1. PPh3, THF 2. 3. LiH, THF ' THF H Harrow_forwardPlease EnCircle or Fill-In your Choice CLEARLY: 21. Please Sketch the intermediates for each step below. Draw the Product which would result from the following series of reactions. Name each Type of Rx: 1. Br2, FeBr3 2. Mg, ether 3. ethylene oxide 4. H₂O+ 5. PBr3 6. Mg, ether 7. 8. H3O+, heat (-H₂O 9. HF ?arrow_forwardCan I please get help with this question. All required information should be in data table.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





