
Concept explainers
(a)
Interpretation: The product formed on thermal electrocyclic ring opening of the given compounds is to be predicted.
Concept introduction: Electrocyclic reactions involve ring opening or ring closure in a conjugated polyene. According to Woodward-Hoffmann rules, the polyene containing even number of bonds in thermal conditions undergoes reaction in conrotatory fashion and polyene containing odd number of bonds undergo reaction in disrotatory fashion.
Answer to Problem 25P
The product formed on thermal electrocyclic ring opening of the compound A is,
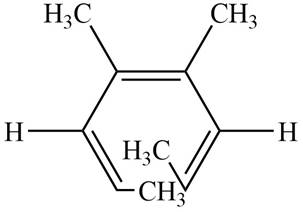
Figure 1
The product formed on thermal electrocyclic ring opening of the compound B is,
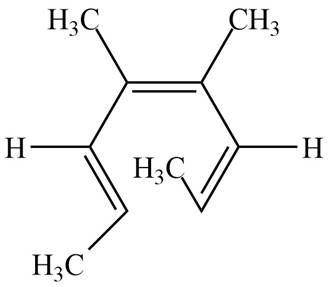
Figure 2
Explanation of Solution
The given compound A is,
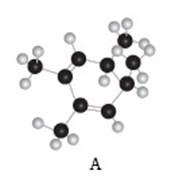
Figure 3
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
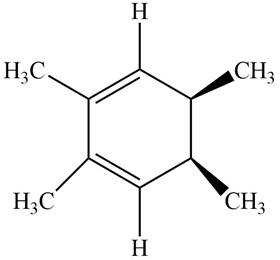
Figure 4
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding
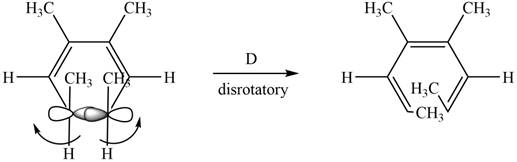
Figure 5
The given compound B is,
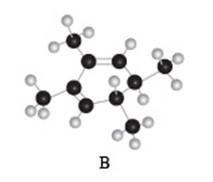
Figure 6
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
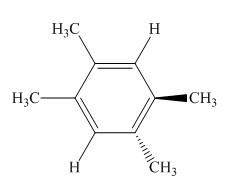
Figure 7
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding chemical reaction is shown below.
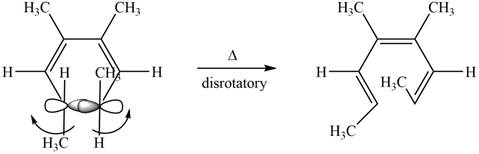
Figure 8
The products formed on thermal electrocyclic ring opening of the compound A and compound B is are shown in Figure 1 and Figure 2.
(b)
Interpretation: The product formed on photochemical electrocyclic ring opening of the given compounds.
Concept introduction: Electrocyclic reactions involve ring opening or ring closure in a conjugated polyene. According to Woodward-Hoffmann rules, the polyene containing even number of bonds in photochemical conditions undergoes reaction in disrotatory fashion and polyene containing odd number of bonds undergo reaction in conrotatory fashion.
Answer to Problem 25P
The product formed on photochemical electrocyclic ring opening of the compound A is,
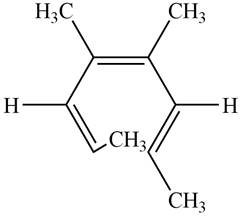
Figure 9
The product formed on photochemical electrocyclic ring opening of the compound B is,
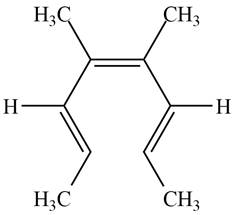
Figure 10
Explanation of Solution
The given compound A is,
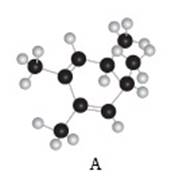
Figure 3
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,
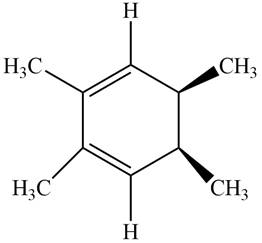
Figure 4
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding chemical reaction is shown below.
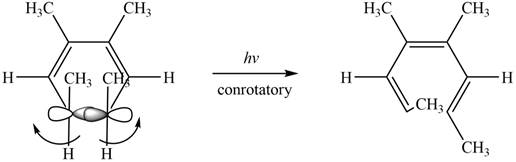
Figure 11
The given compound B is,
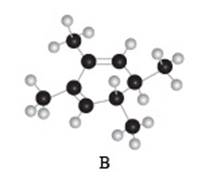
Figure 6
Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure is,

Figure 7
The given compound undergoes thermal electrocyclic ring opening to form product with three pi bonds. According to Woodward-Hoffmann rules, the ring opening takes place in disrotatory fashion. The atomic orbitals of the carbon atoms whose sigma bond is broken rotates in opposite direction. The corresponding chemical reaction is shown below.
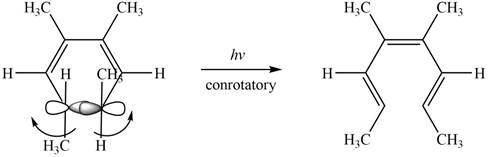
Figure 12
The products formed on photochemical electrocyclic ring opening of the compound A and compound B is are shown in Figure 9 and Figure 10.
Want to see more full solutions like this?
Chapter 25 Solutions
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
- 10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forwardPredicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward4. The major product from treatment of 2-propanol with the Jonesreagent is ___.A. acetone; B. none of the other answers is correct C. propene; D.propanoic acid; E carbon dioxide. Please include all steps! Thank you!arrow_forward
- 7. All of the following compounds that are at the same oxidation levelare ___.u. methyl epoxide, v. propyne, w. propanal, x. propene,y. 2,2-dihydroxypropane, z. isopropanol?A. u,v,w,y; B. u,v,w; C. v,w,y,z; D. v, z; E. x,y,z Please include all steps. Thank you!arrow_forward9. Which one of the following substituents is the worst leaving group inan SN2 reaction? A. -NH2; B. -OH; C. –F; D. NH3; E. H2O Please include all steps. Thanks!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 2.5 × 105: CO(g) + H2O(g) CO2(g) + H2(g) Use this information to complete the following table. Suppose a 7.0 L reaction vessel is filled with 1.7 mol of CO and 1.7 mol of H2O. What can you say about the composition of the mixture in the vessel at equilibrium? What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. CO2(9)+H2(g) CO(g)+H₂O(g) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 CO(g)+3H2O(g) = 3 CO2(g)+3H2(g) There will be very little CO and H2O. x10 There will be very little CO2 and H2. 000 Neither of the above is true. K = ☐ K = ☐ 18 Ararrow_forward
- 8. When ethane thiol is treated with hydrogen peroxide the product is___.A. ethane disulfide; B. diethyl sulfide; C. ethane sulfoxide; D. ethanesulfate; E. ethyl mercaptan. Please include all steps. Thanks!arrow_forward5. The major product of the three step reaction that takes place when 1-propanol is treated with strong acid is?A. dipropyl ether; B. propene; C. propanal; D. isopropyl propyl ether;E. 1-hexanol Please include all steps. Thank you!arrow_forward6. The formula of the product of the addition of HCN to benzaldehydeis ___.A. C8H7NO; B. C8H6NO; C. C14H11NO; D. C9H9NO; E. C9H8NO Please include all steps. Thank you!arrow_forward
- Predicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid K K a name formula name formula nitrous acid HNO2 4.5×10 hydroxylamine HONH2 1.1 × 10 8 hypochlorous acid HCIO 8 3.0 × 10 methylamine CH3NH2 | 4.4 × 10¯ 4 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. 0.1 M KCIO solution PH choose one 0.1 M NaNO2 0.1 M CH3NH3Br 0.1 M NaBr choose one ✓ choose one v ✓ choose one 1 (lowest) ☑ 2 3 4 (highest)arrow_forwardFor this Orgo problem, don't worry about question 3 below it. Please explain your thought process, all your steps, and also include how you would tackle a similar problem. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 0.84: H2(g) + 2(g) 2 HI(g) = Use this information to complete the following table. Suppose a 34. L reaction vessel is filled with 0.79 mol of HI. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little H2 and 12. ☐ x10 There will be very little HI. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 HI(g) H₂(9)+12(9) K = What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 H2(g)+212(9) 4 HI(g) K = ☐ ☑arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





