
(a)
Interpretation:
Which H would most likely be abstracted by bromine radical
Concept introduction:
The H atom with the weakest bond and leads to the formation of stable ion is the most reactive H atom i.e. likely is abstracted by radical. The H atom attached to the carbon with more number of alkyl group form weaker bond
Answer to Problem 25.37P
The H atom which is likely to be abstracted by bromine radical
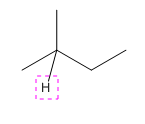
Explanation of Solution
The given compound is:
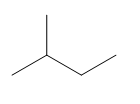
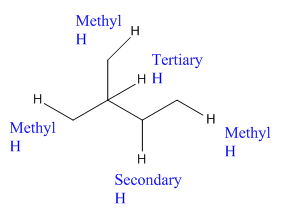
In the above compound there are three types of C atom; methyl, secondary and tertiary carbon atoms. Hydrogen atoms attached to respective carbons are also methyl H, secondary H and tertiary H atoms.
The H atom attached to the carbon with more number of alkyl group form weaker bond

Which H would most likely be abstracted by bromine radical
(b)
Interpretation:
Which H would most likely be abstracted by bromine radical
Concept introduction:
The H atom with the weakest bond and leads to the formation of stable ion is the most reactive H atom i.e. likely is abstracted by radical. The H atom attached to the carbon with more number of alkyl group form weaker bond
Answer to Problem 25.37P
The H atom which is likely to be abstracted by bromine radical
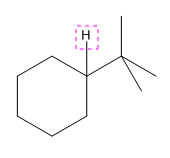
Explanation of Solution
The given compound is:
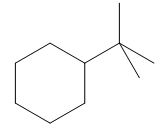
In the above compound there are three types of C atom; methyl, secondary and tertiary carbon atoms. Hydrogen atoms attached to respective carbons are also methyl H, secondary H and tertiary H atoms.
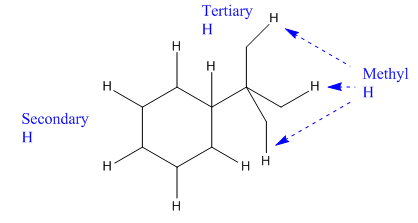
The H atom attached to the carbon with more number of alkyl group form weaker bond

Which H would most likely be abstracted by bromine radical
(c)
Interpretation:
Which H would most likely be abstracted by bromine radical
Concept introduction:
The H atom with the weakest bond and leads to the formation of stable ion is the most reactive H atom i.e. likely is abstracted by radical. The H atom attached to the carbon with more number of alkyl group form weaker bond
Answer to Problem 25.37P
The H atom which is likely to be abstracted by bromine radical
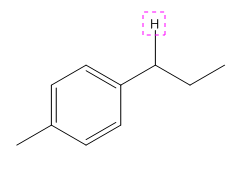
Explanation of Solution
The given compound is:
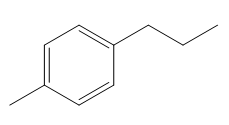
In the above compound there are four types of C atom; methyl, secondary, secondary benzylic and
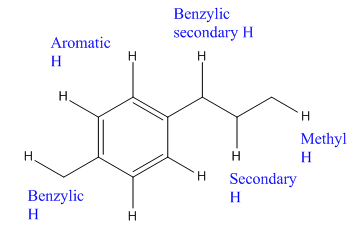
The H atom attached to the carbon with more number of alkyl group form weaker bond

Here, the another benzylic radical could form by abstracting a H atom from the benzylic
Which H would most likely be abstracted by bromine radical
(d)
Interpretation:
Which H would most likely be abstracted by bromine radical
Concept introduction:
The H atom with the weakest bond and leads to the formation of stable ion is the most reactive H atom i.e. likely is abstracted by radical. The H atom attached to the carbon with more number of alkyl group form weaker bond
Answer to Problem 25.37P
The H atom which is likely to be abstracted by bromine radical
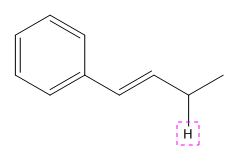
Explanation of Solution
The given compound is:
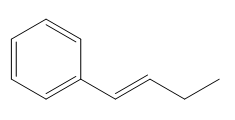
In the above compound there are three types of C atom; methyl, allylic, and aromatic carbon atoms. Hydrogen atoms attached to respective carbons are also methyl H, allylic, and aromatic H atoms.
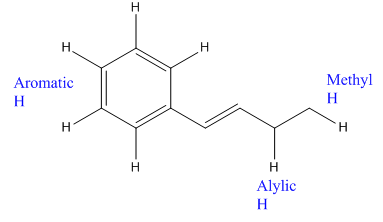
The H atom attached to the carbon with more number of alkyl group form weaker bond

Which H would most likely be abstracted by bromine radical
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry: Principles And Mechanisms (second Edition)
- Consider the following nucleophilic substitution reaction. The compound listed above the arrow is the solvent for the reaction. If nothing is listed over the arrow, then the nucleophile is also the solvent for the reaction. Part 1 of 2 Br CH,CN + I¯ What is the correct mechanism for the reaction? Select the single best answer. @SN2 ○ SN 1 Part: 1/2 Part 2 of 2 Draw the products for the reaction. Include both the major organic product and the inorganic product. If more than one stereoisomer is possible, draw only one stereoisomer. Include stereochemistry where relevant. Click and drag to start drawing a structure. X હૈarrow_forward20.33 Think-Pair-Share (a) Rank the following dienes and dienophiles in order of increasing reactivity in the Diels-Alder reaction. (i) CO₂Et (ii) COEt || CO₂Et MeO MeO (b) Draw the product that results from the most reactive diene and most reactive dienophile shown in part (a). (c) Draw a depiction of the orbital overlap involved in the pericyclic reaction that oc- curs between the diene and dienophile in part (b). (d) Is the major product formed in part (b) the endo or exo configuration? Explain your reasoning.arrow_forward20.40 The following compound undergoes an intramolecular Diels-Alder reaction to give a tricyclic product. Propose a structural formula for the product. CN heat An intramolecular Diels-Alder adductarrow_forward
- What is the reaction mechanism for this?arrow_forwardWhat is the reaction mechanism for this?arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. + Drawing Arrows CH3ONA, CH3OH heat : Br:O Na → H H Br Na + H H H H H :0: .H + Undo Reset Done Q CH3 Drag To Pan +arrow_forward
- What is the reaction mechanism for this?arrow_forward20.19 Predict the structure of the major 1,2-addition product formed by reaction of one mole of Cl₂ with 3-methylenecyclohexene. Also predict the structure of the 1,4-addition product formed under these conditions. 20.20 Which of the two molecules shown do you expect to be the major product formed by 1,2-addition of HCI to cyclopentadiene? Explain. Cyclopentadiene + HC 3-Chlorocyclopentene (racemic) or 4-Chlorocyclopentene (racemic)arrow_forward20.35 Propose structural formulas for compounds A and B and specify the configuration of compound B. EtO₂C 250°C C14H2004 CO₂Et 1. Oso, then NaHSO3 2. HIO4 C14H2006 A Barrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





