
Concept explainers
a) CH3CHNH2 or CH3CH2 CONH2
Interpretation:
The levis concept (due to GN levis) which is the most general concept of a base defines a base as any species which is sufficient electron rich and contains at last one or more unshared electron pains available for donation, which subsequent electronic interaction or an apparent band formation with an acid, an electron deficient species.
Answer:
Ethylamine CH3CH2NH2 is more basic the amide (ethylamide) CH3CH2CoNH2.
Explanation:
Thus basicity is an attribute defecting the extent of overlapping of this done electron pain donation. Quantitative it is measured by this extent; and also, any structural or chemical environmental factor that tends to increase or active increase the extent of available of donation of this electron pain(s) to a nembutal acid is acid to enhance the basicity of a levis base.
On the basis of the above, we examine what structure or other factor in the two given molecules amide and amine lead then to the bases at all in the fast; and what facter causes them to be which laser copying strengths.
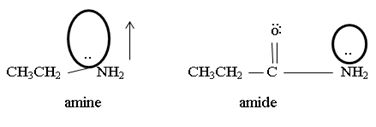
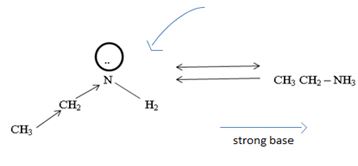
1) Fact I, Both are nitrogenous bases ie the basicity is due, intermediate to the problem of an unshared electron pair on the nitrogen atom of the –NH2-amine group. Thus more available then electron pair of on incident acid, the stronger is the base. Closely, the base strength all the diminished of any factor causes this electron pair to the less available for be genetics within appropriate acid.
2) Fact II, → considered ethylamine and ethanamide.
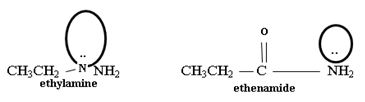
Ethanamide is much stronger base because of the electron releasing positive inductive +g effect of the ethyl (alkyl) group makes the N lone pair more available to any incident proton H+.
Havens is ethanamide, true is an incident stability caused by delocalization of the nitrogen line-pair electron through orbital overall with the carbonyl group. In resonance terms, amides are more states, and also then reactive than amides because this are hybrids of two resonance forms this amide resonance stabilization is lost when the nitrogen atom is protonated. So petroleum is disfavored. The following structural resonance form and electron density diagrams shown a significants reduced electron density on the amide nitrogen.
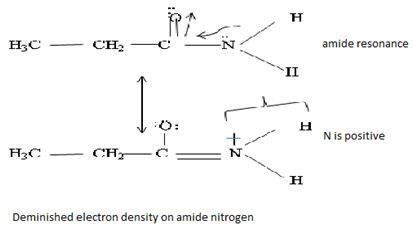
Oxygen – pauling electrons acids = 3.44
Nitrogen – pauling electrons acids = 3.04
Further the very electrons acid values of N is O, specifies the electron delocalization form N to O in amides, and consolidate the structure resonance forms.
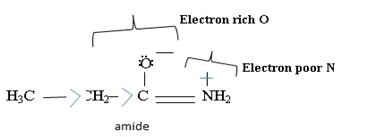
Thus, the very factor that lands amides then reactive also causes their reduced basicity than
Conclusion:
Form the above teach of relative basicity, it comes urtherit saying that population bile basics acids are governed by structural features in substances and chemical environments.
b) NCOH or CH3NH2
Interpretation:
As the outset, let it be said that acidity and basicity are measures of equilibrium (
Sodium hyduxide is a potentates much stronger base than methylamine.
Explanation:
The stronger this officials the more is the ease of proton uptake, and more is the hold on this proton; thus the stronger the species as a base.
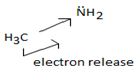
Organic bases are potencies much stronger bases than organic bases, v3 amines. Thus NCOH is a much stronger base then CH3NH2 an methylamine CH3NH2, the basicity is attributed to the presence of the unshared electron paid on the nitrogen of the amino group. The extent of available of this electron pair is increased by the inductive release of electron dentist by the methyl group. This miles methylamine strong organic base, but ten, then NAOH, the inorganic base.
Conclusion:
Sodium hyduxide is a potentates much stronger base than methylamine.
c) CH3NHCH3 or pyridine
Interpretation:
In organic bases, the basics is exhibited to the extent of available of a lone pair(s) of electron an nitrogen atom of the amino group, the more available then electron pair for uptake of a proton, the stronger the base.
Explanation:
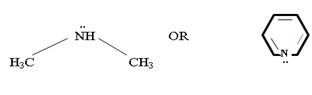
In dimethyl amine, inductive release of electrons by the two methyl groups increase the electron density on the nitrogen atom to an appropriate degree. The compound is thus a very strong base, convenes, in the heterocyclic amine pyridine, the line electron pair on nitrogen is lost by amides delocalization into the benzene ring and nitrogen causes c positive change. Thus it is a very week base.
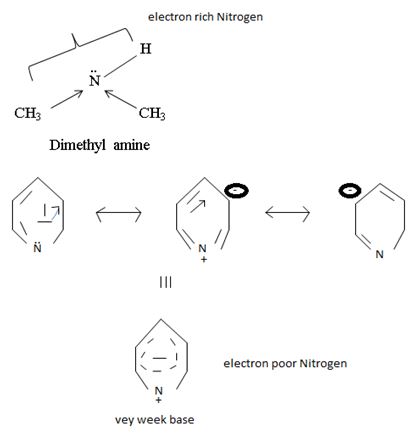
Conclusion:
Basicity in organic amines thus is a measure of the extent of availability of an unshared electron pair on the amines stronger amines than both
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

Chapter 24 Solutions
EP ORGANIC CHEMISTRY,24 MONTH-OWLV2
- Be sure to answer all parts. The following alkyne is treated with 03 followed by H₂O. Part 1: How many different compounds are formed in this process? 1 Part 2 out of 2 Draw the product of the reaction. draw structure ...arrow_forwardMany fireworks use magnesium to burn, which releases a significant amount of energy. The heat released causes the oxide to glow, emitting white light. The color of this light can be changed by including nitrates and chlorides of elements that emit in the visible region of their spectra. One such compound is barium nitrate, which produces a yellow-green light. Excited barium ions generate light with wavelengths of 487 nm, 524 nm, 543 nm, and 578 nm. For each case, calculate: (a) the change in energy (in electron volts) of a barium atom and (b) the molar change in energy (in kilojoules per second).arrow_forwardClouds of hot, luminous interstellar hydrogen gas can be seen in some parts of the galaxy. In some hydrogen atoms, electrons are excited to quantum levels with n = 100 or higher. (a) Calculate the wavelength observed on Earth if the electrons fall from the level with n = 100 to one with n = 2. (b) In what series would this transition be found? (c) Some of these high-energy electrons fall into intermediate states, such as n = 90. Would the wavelengths of a transition from the state with n = 100 to one with n = 90 be longer or shorter than those in the Balmer series? Explain your answer.arrow_forward
- In the spectroscopic technique known as photoelectron spectroscopy (PES), ultraviolet radiation is directed at an atom or molecule. Electrons are ejected from the valence shell and their kinetic energies are measured. Since the energy of the incident ultraviolet photons is known and the kinetic energy of the ejected electron is measured, the ionization energy, I, can be deduced because total energy is conserved. (a) Show that the velocity, v, of the ejected electron and the frequency, n, of the incident radiation are related by hv = I + (1/2)mv^2? (b) Use this relation to calculate the ionization energy of a rubidium atom, knowing that light of wavelength 58.4 nm produces electrons with a velocity of 2,450 km/s Recall that 1 J = 1 kg.m^2/s^2arrow_forwardI) In Millikan's experiment, each droplet observed by the technicians contained an even number of electrons. If they had been unaware of this limitation, how would it have affected their report of an electron's charge?II) Millikan measured the charge of an electron in electrostatic units, esu. The data he collected included the following series of charges found on oil drops: 9.60 X 10^-10 esu, 1.92 X 10^-9 esu; 2.40 X 10^-9 esu; 2.88 X 10^-9 esu; and 4.80 X 10^-9 esu. (a) From this series, find the probable charge of the electron in electrostatic units. (b) Estimate the number of electrons in an oil drop with a charge of 6.72 X 10^-9 esu. The actual charge (in Coulombs) of an electron is 1.602 X 10^-19 C. What is the relationship between esu and Coulombs?arrow_forwardmy ccc edu - Search X Quick Access X D2L Homepage - Spring 2025 x N Netflix X Dimensional Analysis - A x+ pp.aktiv.com Q ☆ X Question 59 of 70 The volume of 1 unit of plasma is 200.0 mL If the recommended dosage for adult patients is 10.0 mL per kg of body mass, how many units are needed for a patient with a body mass of 80.0 kg ? 80.0 kg 10.0 DAL 1 units X X 4.00 units 1 1 Jeg 200.0 DAL L 1 units X 200.0 mL = 4.00 units ADD FACTOR *( ) DELETE ANSWER RESET D 200.0 2.00 1.60 × 10³ 80.0 4.00 0.0400 0.250 10.0 8.00 & mL mL/kg kg units/mL L unit Q Search delete prt sc 111 110 19arrow_forward
- Identify the starting material in the following reaction. Click the "draw structure" button to launch the drawing utility. draw structure ... [1] 0 3 C10H18 [2] CH3SCH3 Harrow_forwardIn an equilibrium mixture of the formation of ammonia from nitrogen and hydrogen, it is found that PNH3 = 0.147 atm, PN2 = 1.41 atm and Pн2 = 6.00 atm. Evaluate Kp and Kc at 500 °C. 2 NH3 (g) N2 (g) + 3 H₂ (g) K₂ = (PN2)(PH2)³ = (1.41) (6.00)³ = 1.41 x 104arrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forward
- H-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forwardDraw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forward
