
(a)
Interpretation:
For the four stereo isomeric aldotetroses, the pair of enantiomers and stereo descriptors is needed to be drawn.
Concept introduction:
- Aldoses are monosaccharides, which contains
Aldehyde group. - The naming of monosaccharides,
- Prefix: aldo or keto indicates whether the monosaccharide is containing aldehyde or
ketone group. - Suffix: ose indicates the carbohydrates
- Root word: indicate the number of carbons.
- Prefix: aldo or keto indicates whether the monosaccharide is containing aldehyde or
- The stereo-descriptor used for monosaccharides is D or L. it is based on the dextrorotatory glyceraldehyde or levorotatory glyceraldehyde.
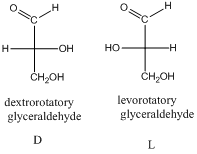
The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Enantiomers are non-super imposable mirror images of each other.
To determine: the structures of pair of enantiomers of stereo isomeric aldotetroses.
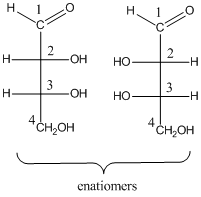
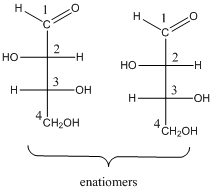
(b)
Interpretation:
For the four stereo isomeric aldotetroses, the pair of enantiomers and stereo descriptors is needed to be drawn.
Concept introduction:
- Aldoses are monosaccharides, which contains Aldehyde group.
- The naming of monosaccharides,
- Prefix: aldo or keto indicates whether the monosaccharide is containing aldehyde or ketone group.
- Suffix: ose indicates the carbohydrates
- Root word: indicate the number of carbons.
- The stereo-descriptor used for monosaccharides is D or L. it is based on the dextrorotatory glyceraldehyde or levorotatory glyceraldehyde.

The stereo-descriptor for other carbohydrates of having more than one chiral center will be evaluated by the location of –OH group (right or left) of farthest chiral carbon from the carbonyl group. Such as,
If the –OH group is located in right side then, the carbohydrate is a D-sugar.
If the –OH group is located in left side then, the carbohydrate is a L-sugar.
- Enantiomers are non-super imposable mirror images of each other.
To identify: the D sugar and L sugar from the four structures of stereo isomeric aldotetroses.
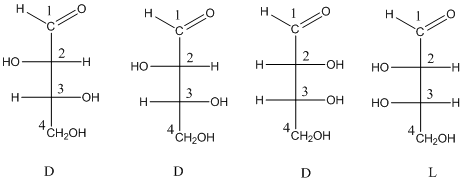
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
ORGANIC CHEMISTRY, WITH SOL. MAN/ STUDY
- Where are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardA mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forward
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





