
Concept explainers
Interpretation:
The structural formulas for the given compounds are to be provided.
Concept Introduction:
In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively.
The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 26P
Solution:
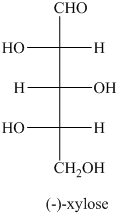
a) The structural formula of
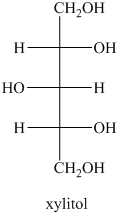
b) The structural formula for the xylitol is
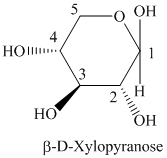
c) The structural formula of
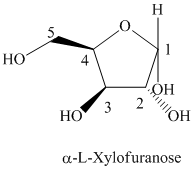
d) The structural formula of
e) The structure of
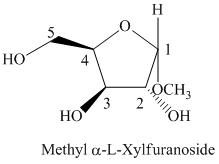
f) The Fischer projection formula for the given compound is shown below.
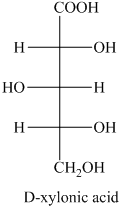
g) The structure of the given compound is shown below.
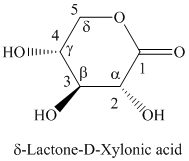
h) The structure of the given compound is shown below.
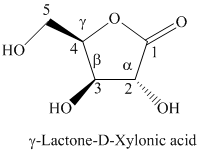
i) The Fischer projection formula for the given compound is shown below.
Explanation of Solution
a) The given structure of
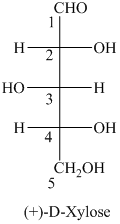
In,
L and D indicate the absolute configuration of a compound. If hydroxy group attached with chiral centre is to the right side and present on the bottom, then the carbohydrate is in D form but if hydroxy group is present to the left side then the carbohydrate is in L-form.
The name of the given compound is
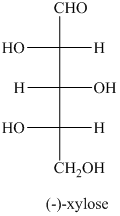
b) The given compound is xylitol.
The alcohol derivative of xylose is xylitol in which the reduction of

c) The given compound is
Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
The given compound is formed when
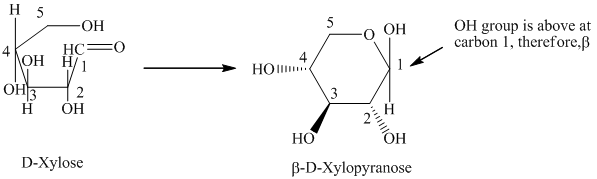
Thus, the structural formula of

d) The given compound is
L and D indicate the absolute configuration of a compound. If hydroxy group attached with chiral centre is to the right side and present on the bottom, then the carbohydrate is in D form but if hydroxy group is present to the left side then the carbohydrate is in L-form.
The given compound is formed when
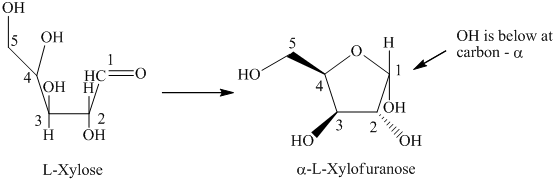
Thus, the structural formula of
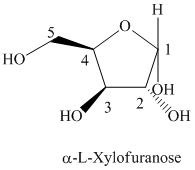
e) The given compound is
The given compound is formed when hydroxyl group is replaced by methoxy group as shown below.
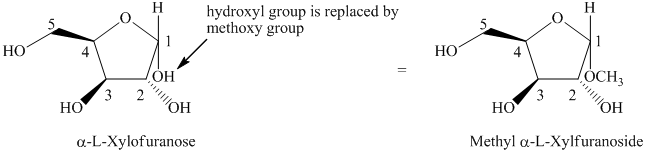
Thus, the structure of
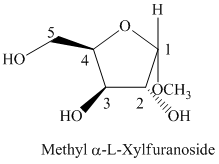
f) The given compound is
The oxidation of
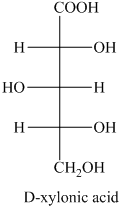
g) The given compound is
Lactones are class of organic compound which contain
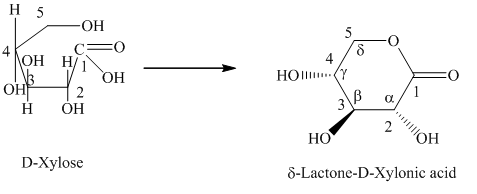
Thus, the structure of the given compound is shown below.

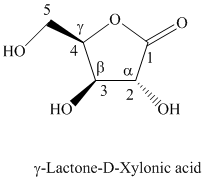
h) The given compound is
Lactones are class of organic compound which contain
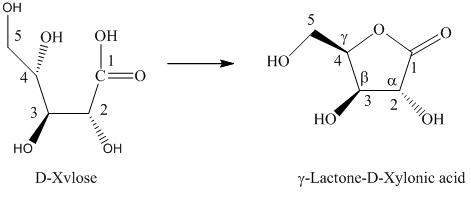
Thus, the structure of the given compound is shown below.
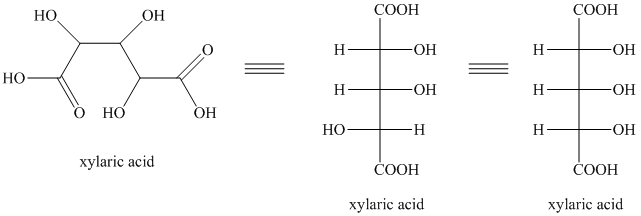
i) The given compound is Xylaric acid.
The oxidation of

Want to see more full solutions like this?
Chapter 24 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
- Can I please get help with identifying these?arrow_forward4. Calculate the pH of a 0.10 M acetic acid (CH3COOH) solution if the Ka of acetic acid = 1.8 x 10-5arrow_forwardDraw the Zaitsev product of the dehydration of this alcohol. + I X 5 OH ざ~ TSOH Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





