
Concept explainers
E. J. Corey’s 1964 total synthesis of α-caryophyllene (essence of cloves) solves a number of problems of construction of unusual-sized rings.
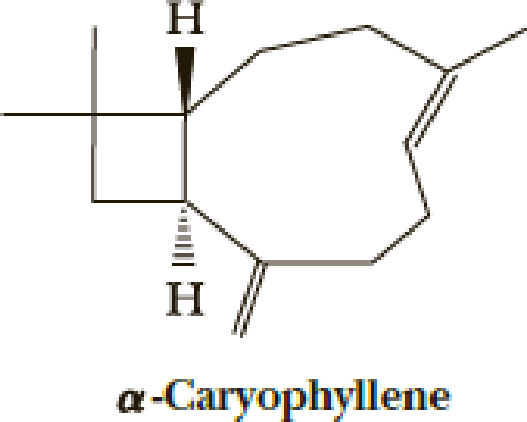
The first step uses an efficient photochemical [2 + 2] reaction. The desired stereochemistry and regiochemistry had been predicted based on model reactions.
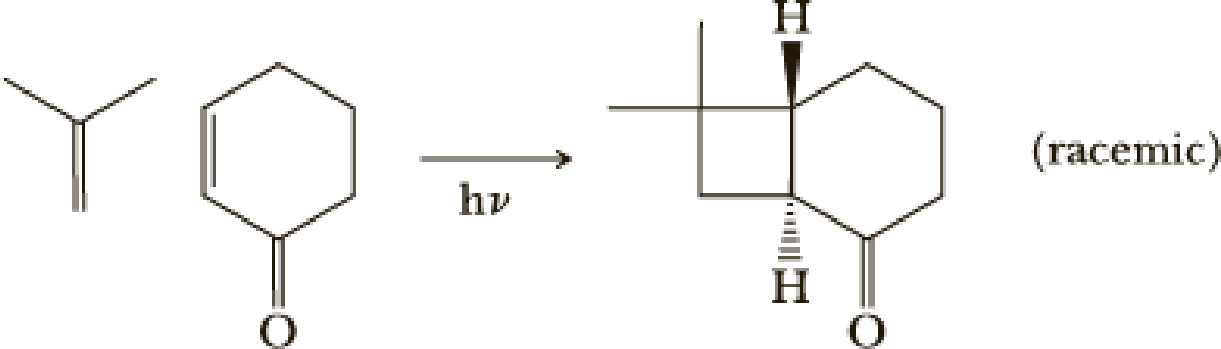
- (a) [2 + 2] Reactions are quite common in photochemical reactions. Would this reaction be predicted to occur in the ground state?
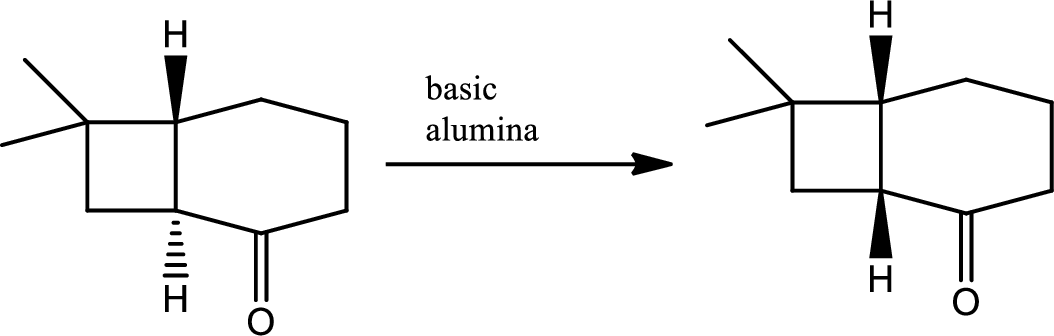
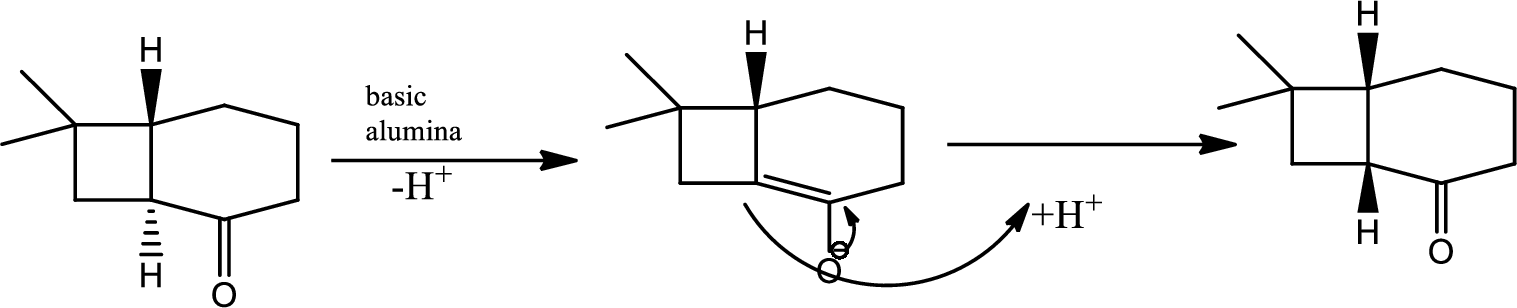
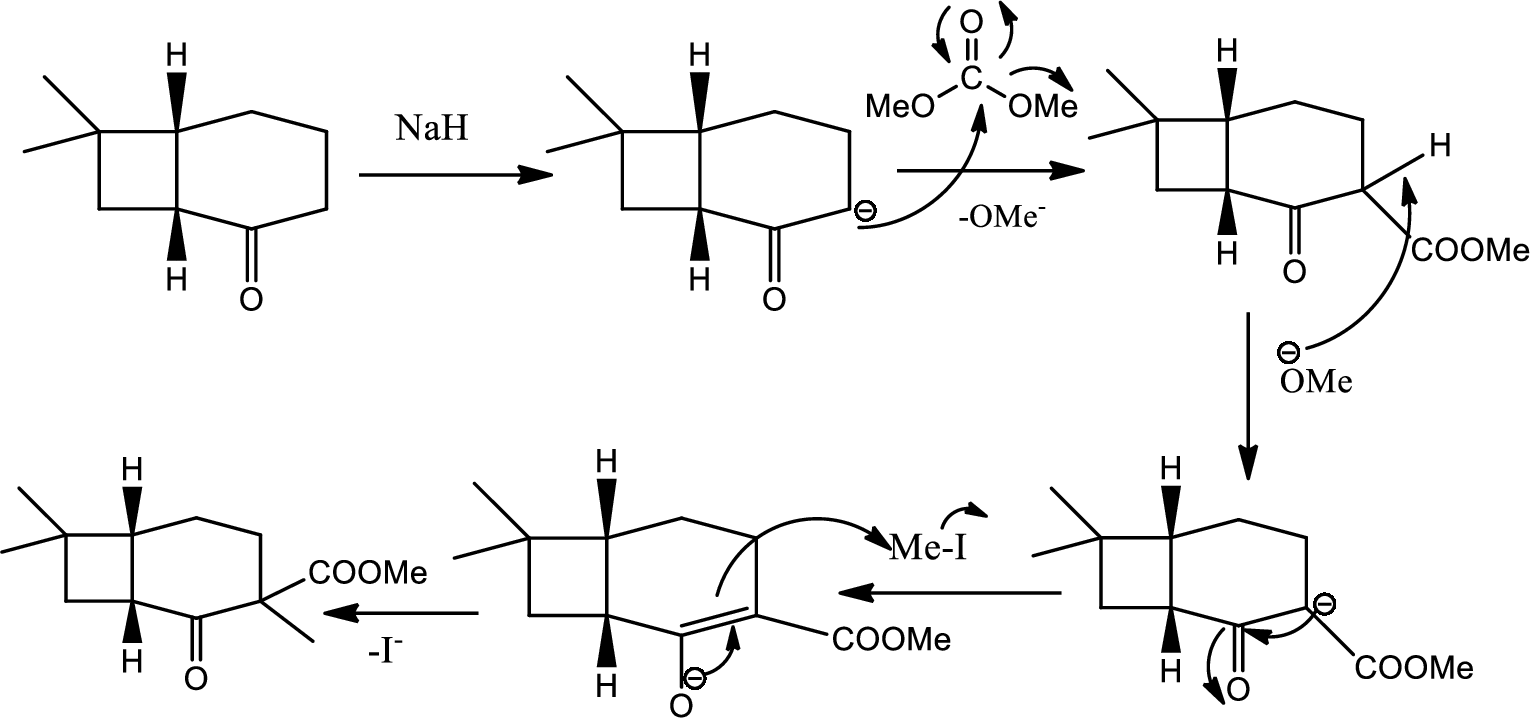
The next steps follow. Basic alumina is a chromatography support that will often act as a base catalyst.
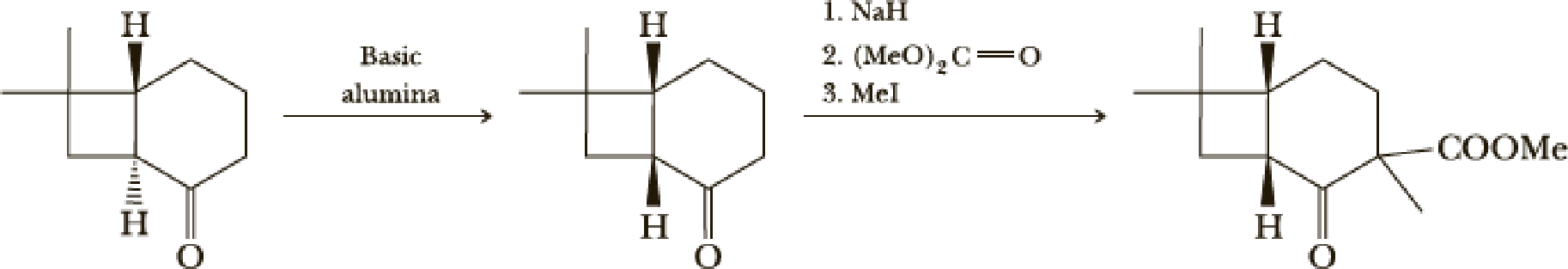
- (b) What is the mechanism of the first step?
- (c) What is the mechanism of the second step?
- (d) Look at later steps in the synthesis. Does the stereochemistry of the added carbomethoxy group matter?
The next steps are shown here.
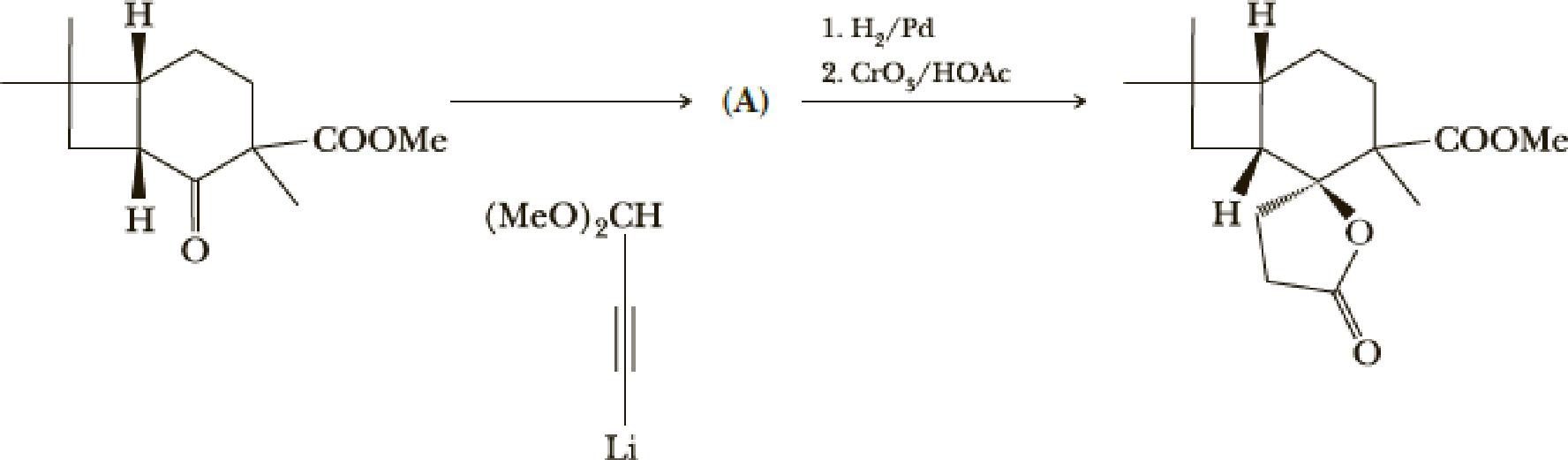
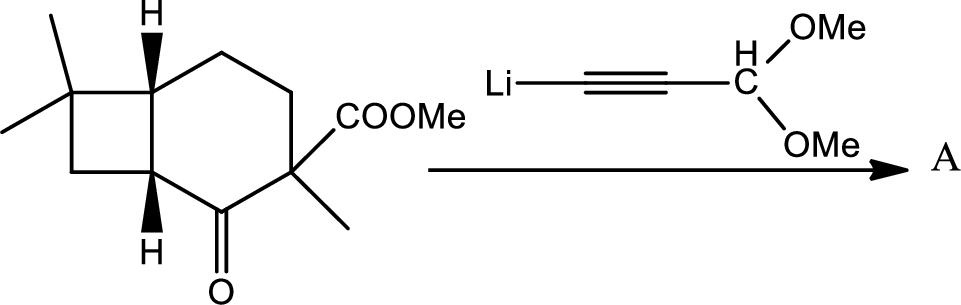
- (e) What is the structure of compound (A)?
- (f) Give a mechanism for the formation of the cyclized product.
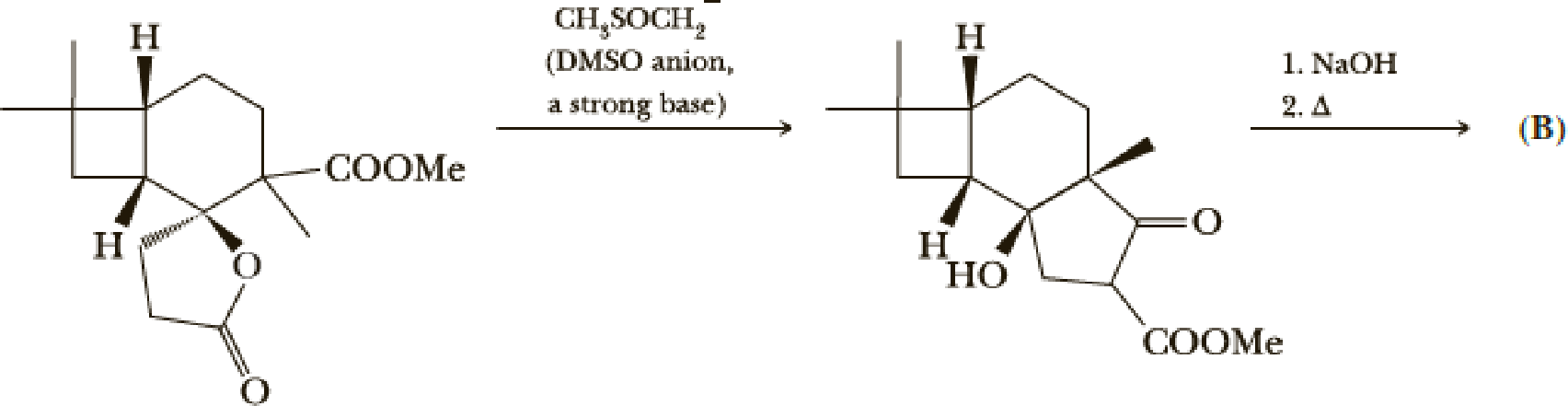
- (g) Give a mechanism for the first step. Hint: Attack on the lactone carbonyl may be the first step.
- (h) Give a structure for product (B).
The following two steps are next.
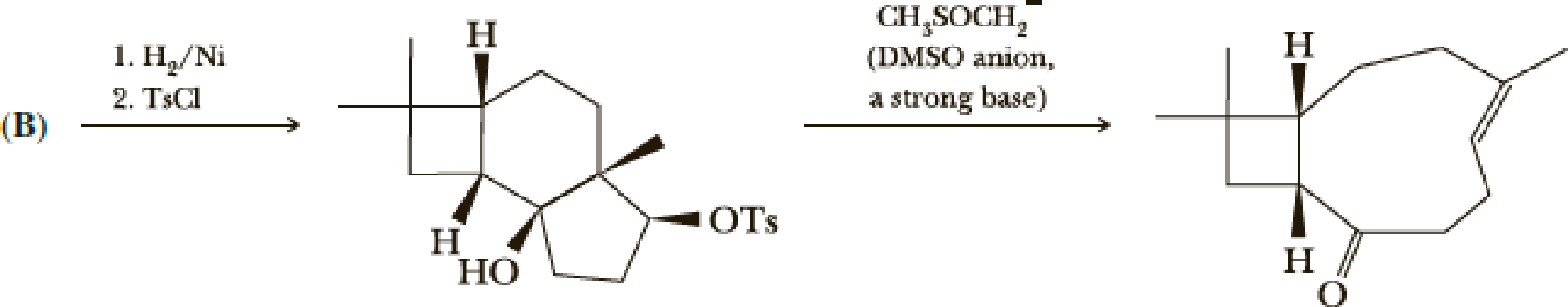
- (i) Show the reactions of (B).
- (j) Write a mechanism for the ring-opening reaction. Hint: Note the presence of an acidic proton and a good leaving group in the molecule.
The synthesis was completed by the following steps.
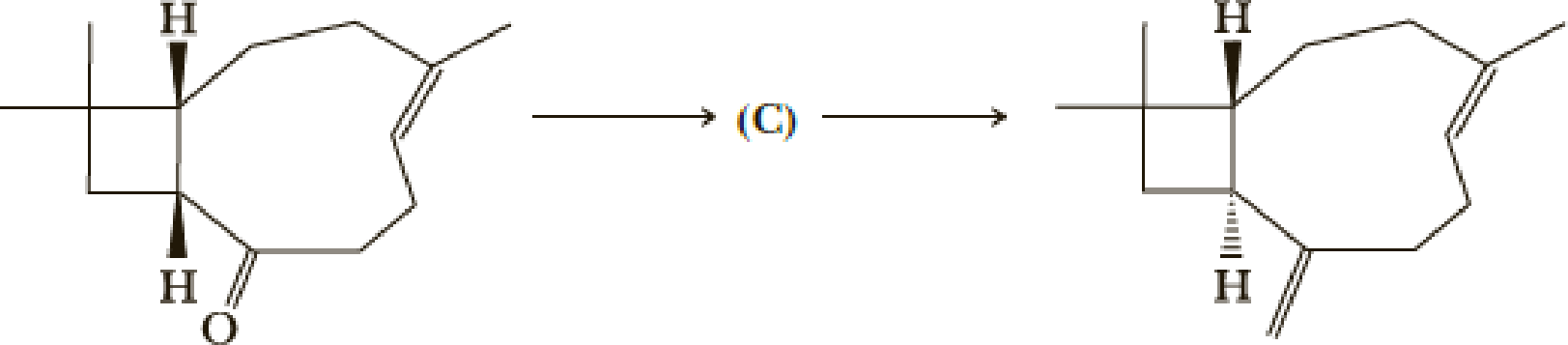
- (k) What is (C)?
- (l) What reagents would you use for these transformations?
(a)
Interpretation:
Whether the
Concept introduction:
Woodward-Hoffmann’s rule:
These are set of rules to determine the geometry and the feasibility of pericyclic reactions. The rules are as follow,
In an open chain system containing
In an open chain system containing
For photochemical condition the electron of HOMO is excited to LUMO and hence the reversal of the terminal symmetry relationship occurs.
Explanation of Solution
According to Woodward Hoffmann’s rule ground state
Ground state it shows
Hence ground state reaction is forbidden.
(b)
Interpretation:
The mechanism of the given step has to be given.
Concept introduction:
Tautomerisation:
Tautomers are structural isomers of a compound that readily interconverts into each other. The process of interconversion is called the tautomerisation and it involves simple proton transfer in intramolecular fashion.
One of the major examples of tautomerisation is keto–enol tautomerism. The mechanism is as follows,
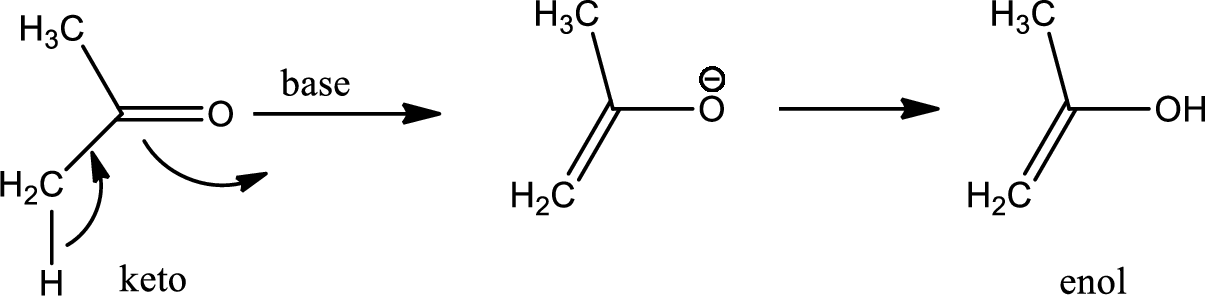
Among these keto and enol keto is more stable due to high bond energy of carbon oxygen double bond (
Explanation of Solution
Here basic alumina acts as base and abstract the enolisable hydrogen from left side because thus the enol form is much more stabilised due to formation of more substituted alkene.
But again the enol form tautomarises to keto form as the bond energy of carbon double bonded oxygen is more than single bonded carbon and oxygen. Thus the proton undergoes backside attack and so this product is formed.
The mechanism is as follows,
(c)
Interpretation:
The mechanism for the given step has to be given.
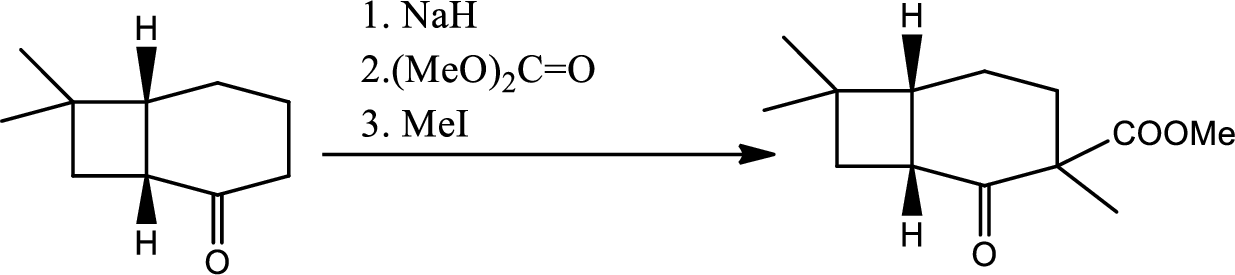
Concept introduction:
Acid-base reaction:
The species that donates proton or accepts lone pair of electrons are called acids and those who accepts proton or donates lone pair of electrons are called base.
The
Explanation of Solution
Here the reaction undergoes acid base reaction followed by
Here
Then again this leaving group
The mechanism is as follows,
(d)
Interpretation:
Whether the stereochemistry of the added carbomethoxy group matters or not that has to be determined.
Concept introduction:
Steric hindrance:
Steric effects are non bondind interactions that influence the shape (conformation) and reactivity of the ions and molecules. Steric effects complement electronic effects which usually dictate shape and reactivity. Steric effects result from repulsive forces between overlapping electron clouds.
Steric hindrance is the consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk.
Explanation of Solution
From the later steps included in the reaction it can be concluded that the added carbomethoxy group’s stereochemistry matters as it is a bulky group and later on the reaction more bulkier rings are formed so it has to be properly oriented to the opposite side to avoid the steric hindrance.
(e)
Interpretation:
The structure of the compound A has to be given for the given reaction,
Concept introduction:
Nucleophilic addition reaction:
Nucleophilic addition reaction can be described as the addition reaction between the chemical compound that is electron deficient and having electron deficeint pi bonds with electron rich chemical compound known as nucleophile that results into disapperance of the pi bond amnd formation of new sigma bonds.
Addition of nucleophile to carbon heteroatom double or triple bonds shows variety as these bonds are polar due to high electrnegetive differences and hence the carbon atom carries a partial positive cahrge thus the carbon atom becomes the electrophilic centre and becomes primary target for nucleophile and thus the reactions occurs is aclled 1,2-nucleophilic addition reaction. The mechanism is as follows,
Organolithium compounds:
Organo lithium compounds are organometallic compounds containing carbon lithium bond. These act as nucleophile and bases also because of the high electronegetive difference between carbon and lithium and the bond becomes polar. Nucleophilic organolithium reagents can add to electrophilic carbonyl double bonds to form carbon-carbon bonds. They react with ketone or aldehyde to give alcohol i.e. they mainly undergoes 1,2-nucleophilic addition reaction
Explanation of Solution
Here the Organolithium compound is used as nucleophile which will attack the carbonyl carbon that behaves as electrophile and reduce it to alcohol. The oxygen lithium bond formed is more polar. The reaction is as follows,
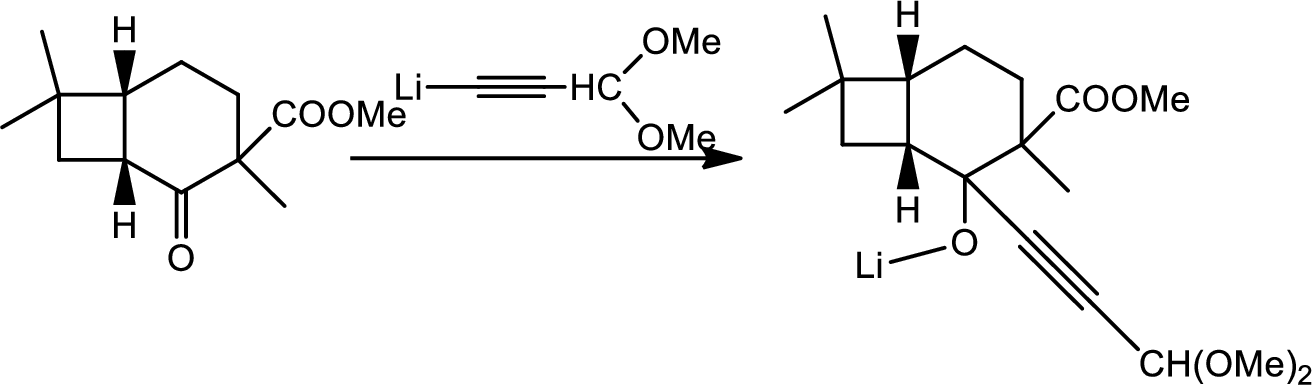
Thus the structure of compound A is,
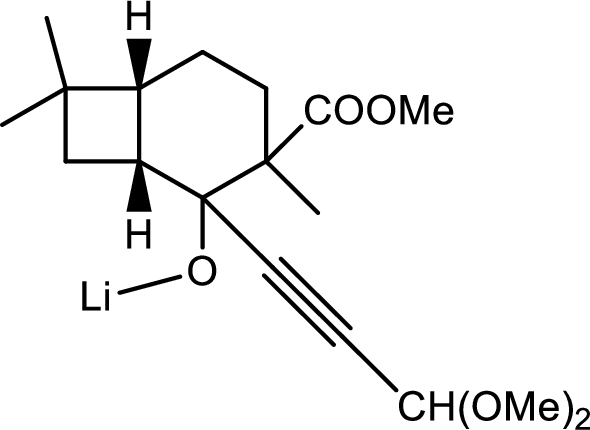
(f)
Interpretation:
The mechanism for the cyclised product given the question has to be given.
Concept introduction:
Reduction of alkyne:
Treatment of an alkyne with
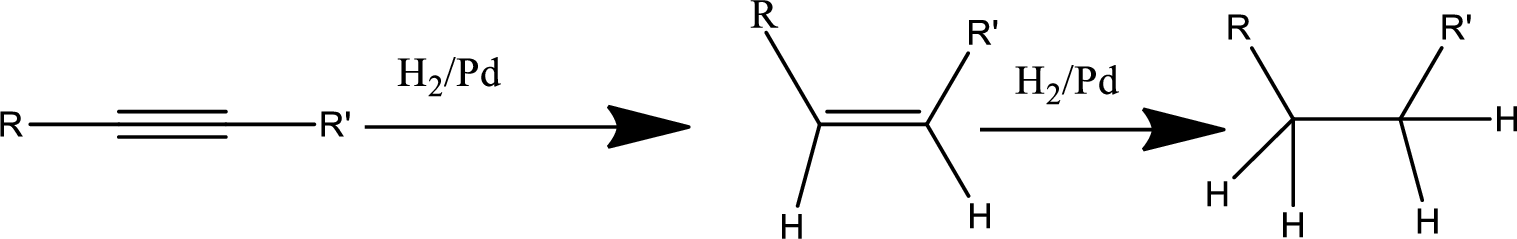
Oxidation by
Chromic acid is used as oxidising agent. It oxidises primary alcohol to acid and it oxidides seconadary alcohol to ketone in presence of acid. The mechanism as follows,
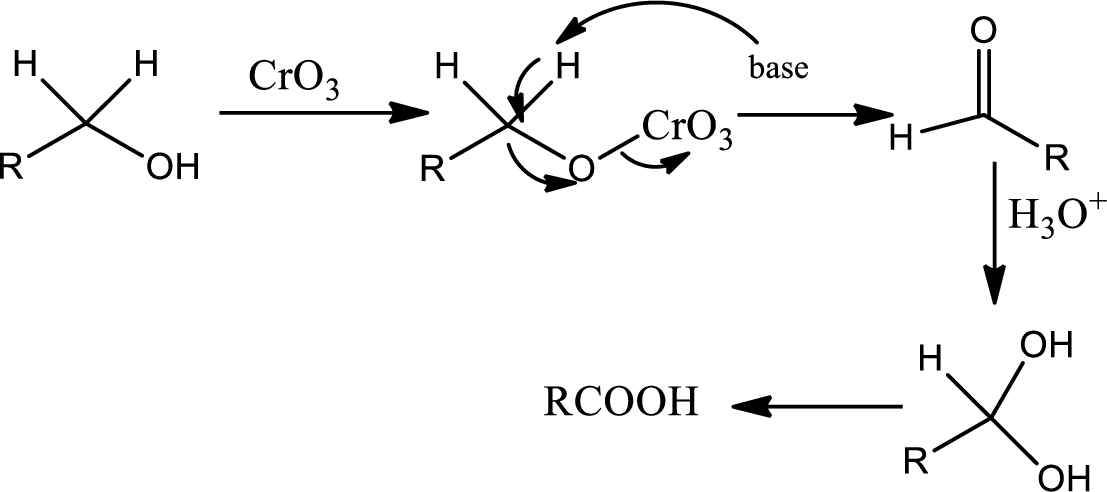
Lactone formation:
Lactones are cyclic carboxylic esters that are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids which takes place spontaneously when th ering that is formed is five or six membered. The reaction pathway is as follows,
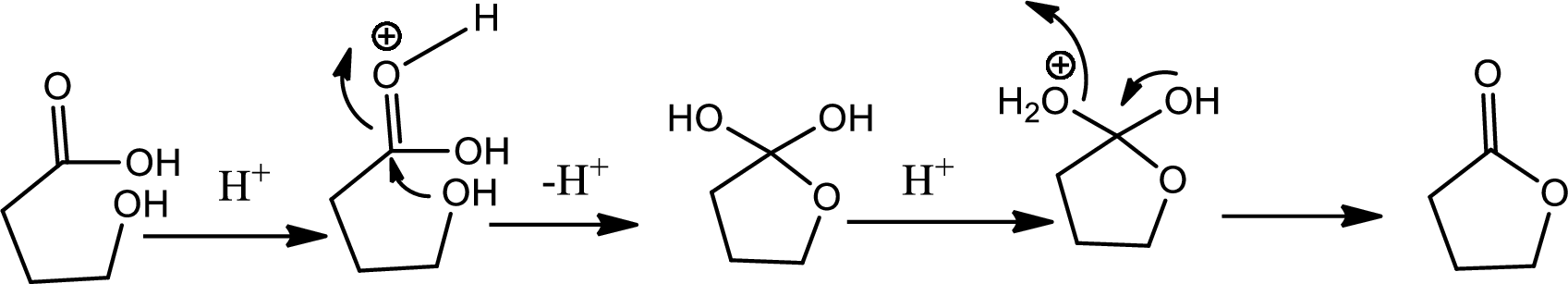
Explanation of Solution
In this pathway of lactone formation 1st the alkyne has been reduced to alkane… the reaction mechanism as follows,
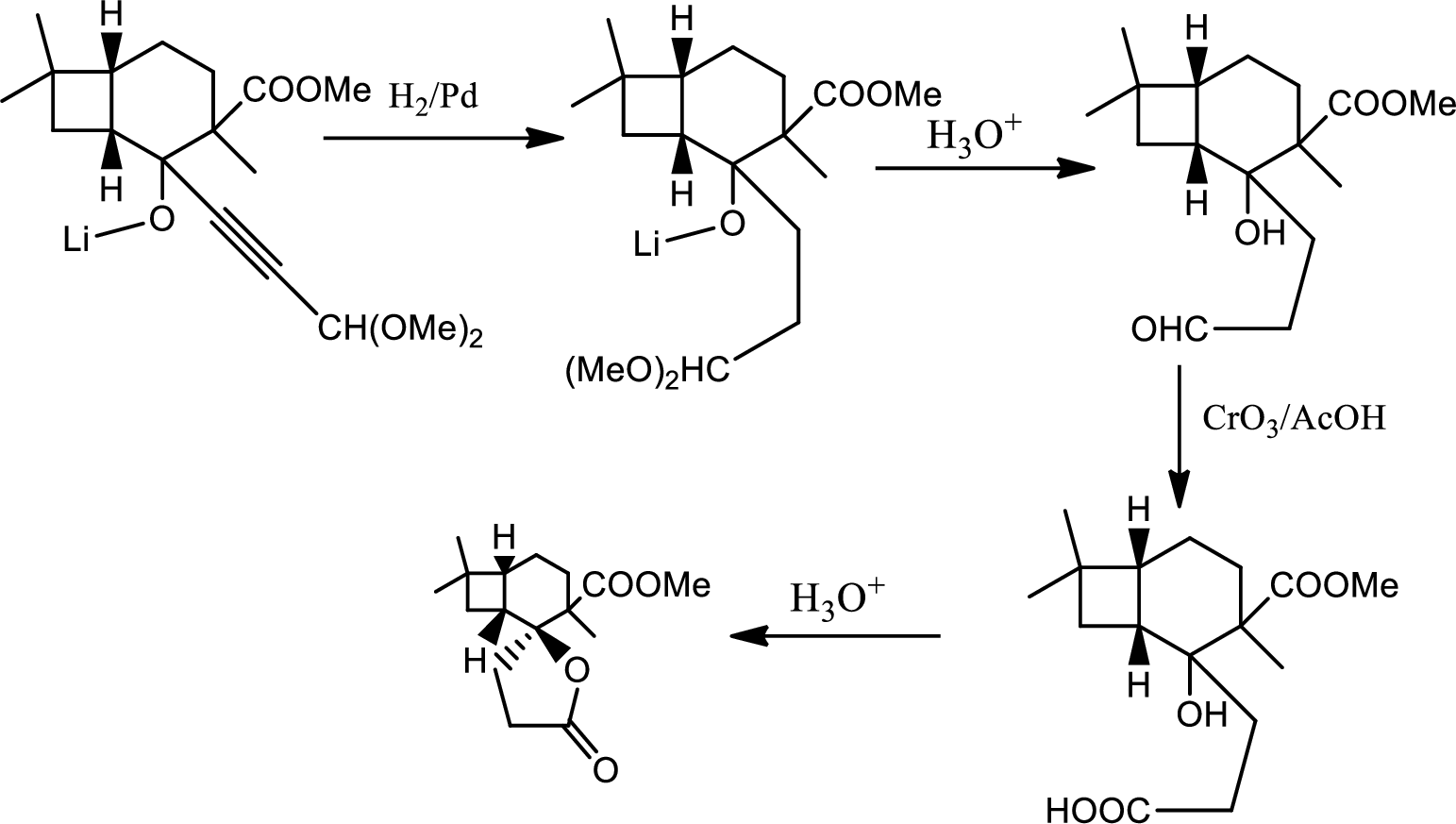
In the 2nd step the orthoester is oxidised to aldehyde which is further oxidised to acid with reaction with
(g)
Interpretation:
Mechanism for the given step has to be given,
Concept introduction:
Acid-base reaction:
The species that donates proton or accepts lone pair of electrons are called acids and those who accepts proton or donates lone pair of electrons are called base.
Explanation of Solution
The mechanism follows as,
(h)
Interpretation:
Structure of product B has to be given.
Concept introduction:
Decarboxylation reaction:
This is the reaction where carboxyl acid group is eliminated along with carbon dioxide.
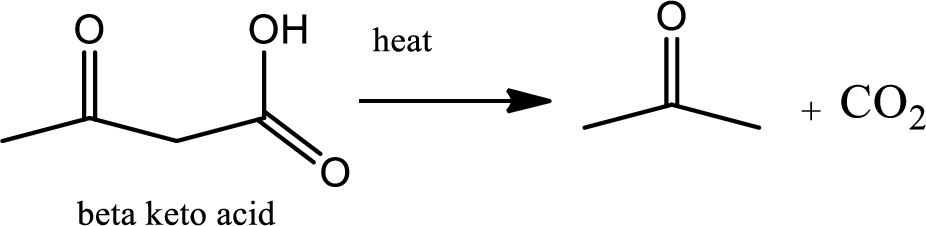
Explanation of Solution
The reaction pathway follows as,
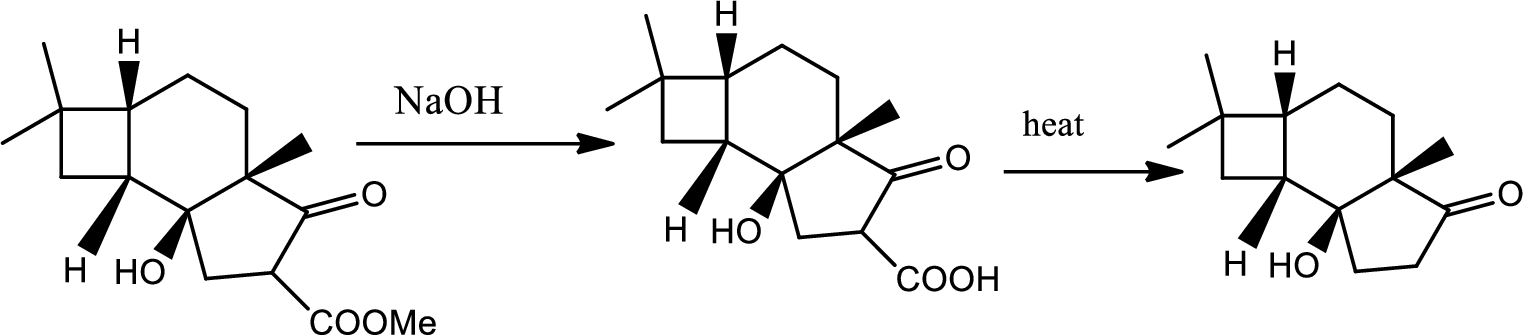
1st the ester is hydrolysed to acid followed by decarboxylation.
Hence the product B is,
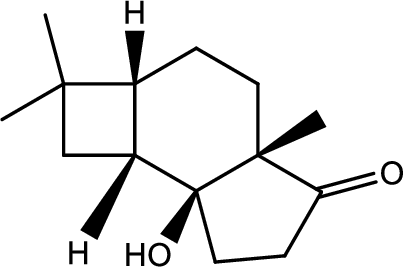
(i)
Interpretation:
The reactions of B for given condition has to be shown.
Concept introduction:
Reduction of ketone:
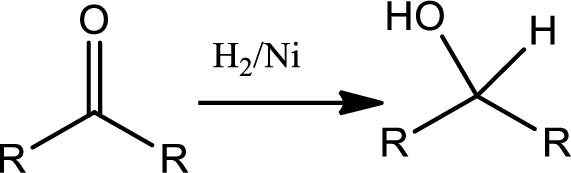
The
Explanation of Solution
The reaction goes as,
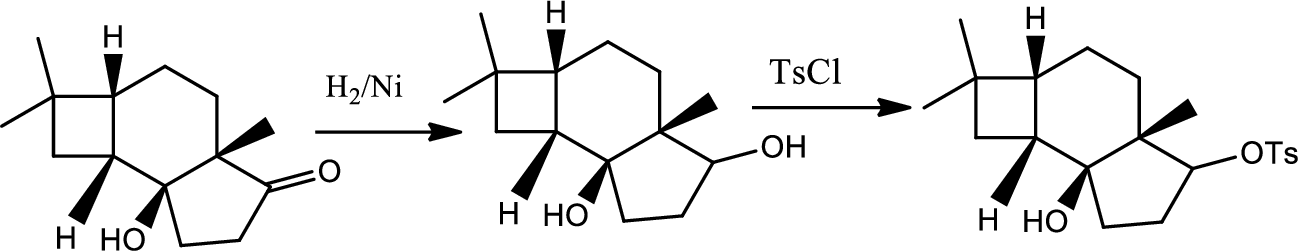
1st the ketone is reduced to alcohol which further undergoes
(j)
Interpretation:
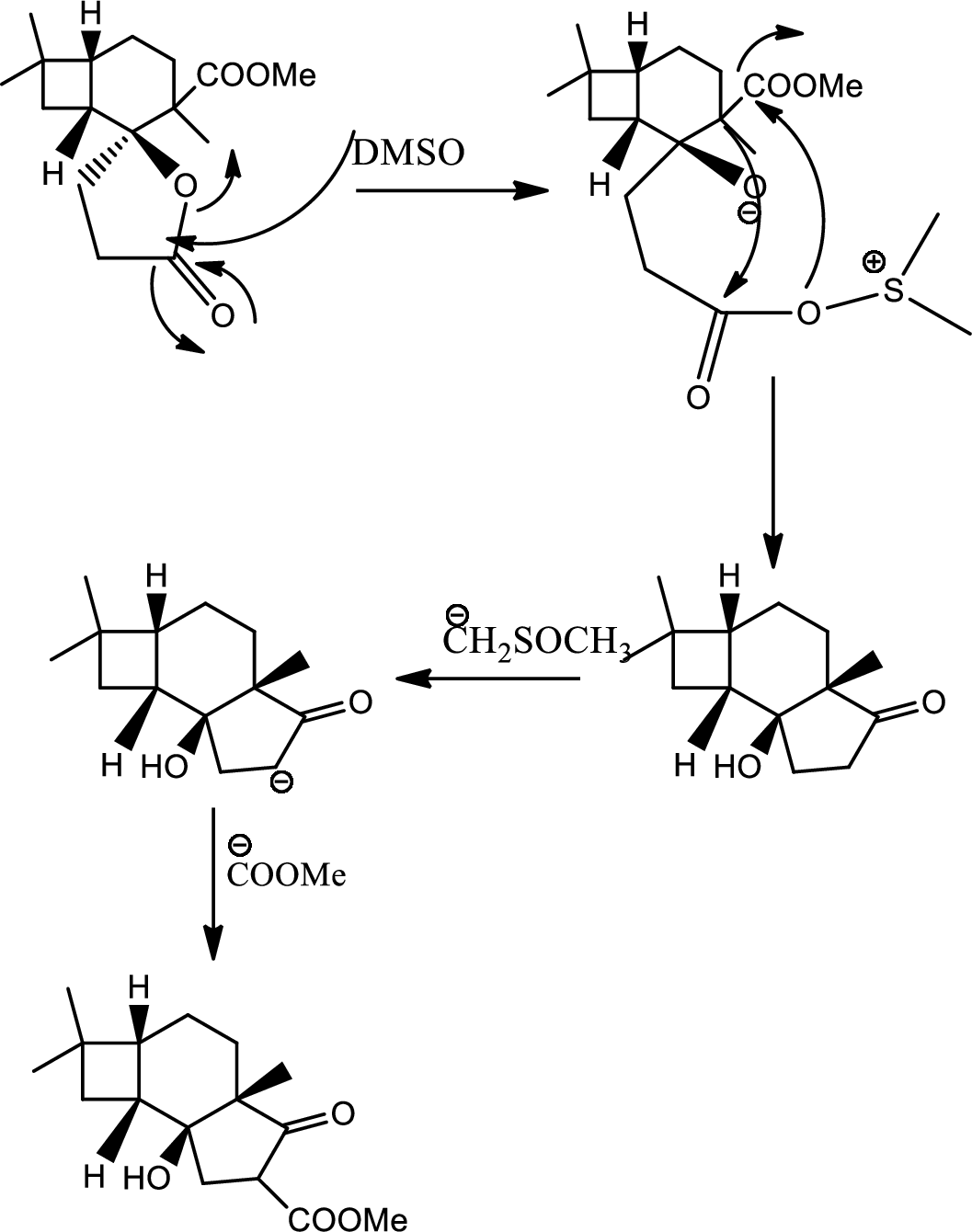
Mechanism of the ring opening reaction has to be shown.
Concept introduction:
Acid-base reaction:
The species that donates proton or accepts lone pair of electrons are called acids and those who accepts proton or donates lone pair of electrons are called base.
Explanation of Solution
DMSO here acts as a strong base and takes up the acidic hydrogen from alcohol. Thus to form strong carbon oxygen double bond the ring opens and
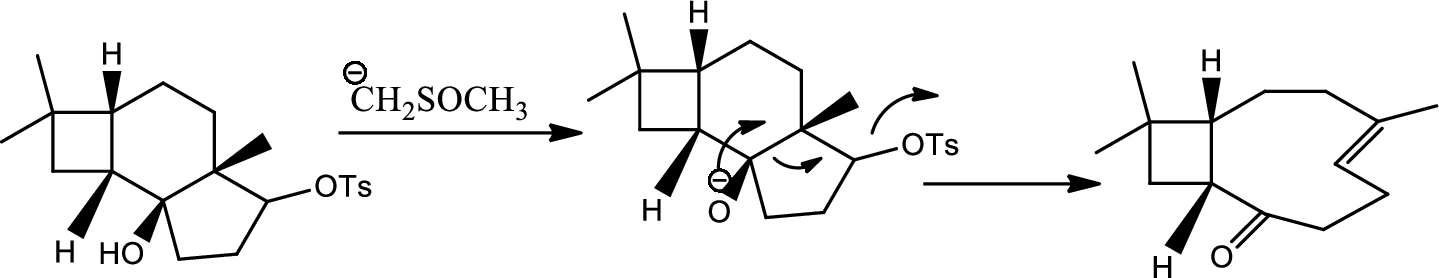
(k)
Interpretation:
The structure of C has to be determined.
Concept introduction:
Tautomerisation:
Tautomers are structural isomers of a compound that readily interconverts into each other. The process of interconversion is called the tautomerisation and it involves simple proton transfer in intramolecular fashion.
One of the major examples of tautomerisation is keto–enol tautomerism. The mechanism is as follows,
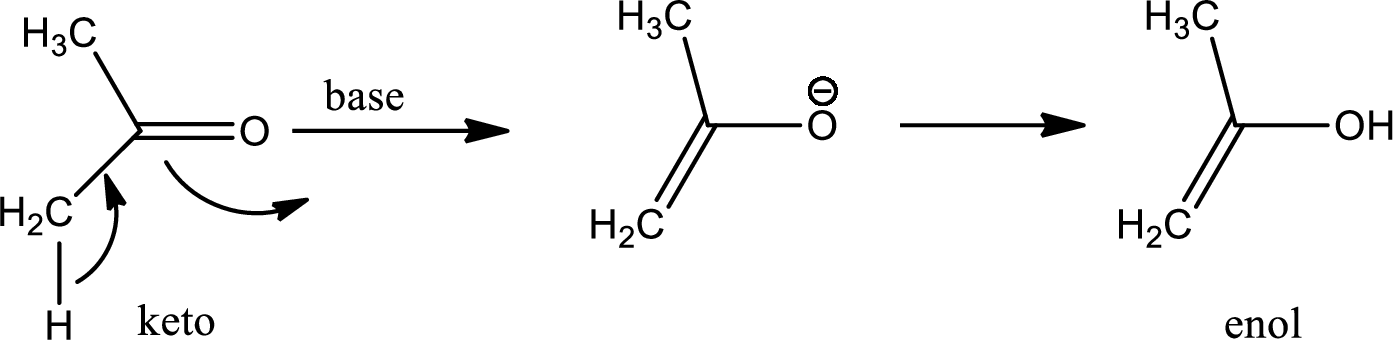
Among these keto and enol keto is more stable due to high bond energy of carbon oxygen double bond (
Explanation of Solution
Here basic alumina acts as base and abstract the enolisable hydrogen from left side because thus the enol form is much more stabilised due to formation of more substituted alkene.
But again the enol form tautomarises to keto form as the bond energy of carbon double bonded oxygen is more than single bonded carbon and oxygen. Thus the proton undergoes backside attack and so this product is formed.
The mechanism is as follows,
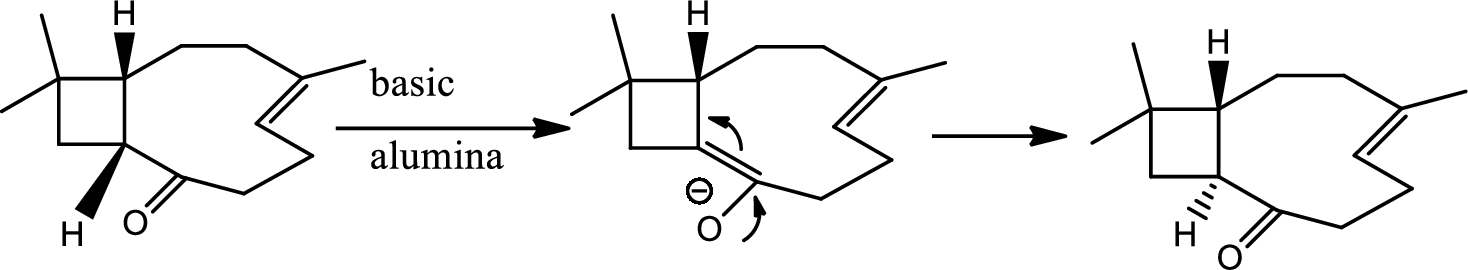
Hence the product C is,
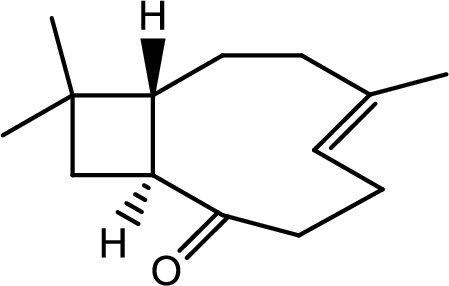
(l)
Interpretation:
The reagents required for the transition given in the question has to be determined.
Concept introduction:
Wittig reaction:
Wittig reaction is the reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide. This is a step up reaction.
Explanation of Solution
The reaction as follows,
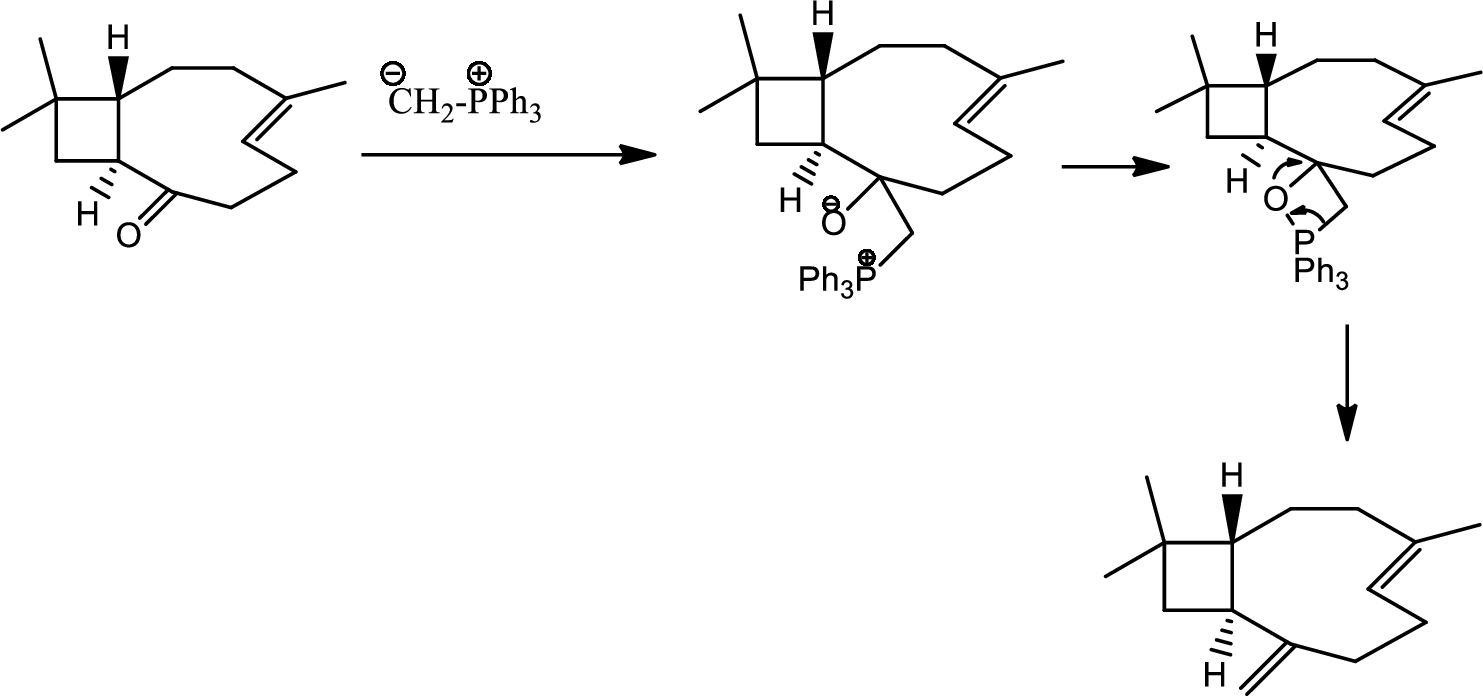
Thus the final product is obtained.
Want to see more full solutions like this?
Chapter 24 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
- in the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forward
- calculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forward
- true or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forward
- the decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward20.00 mL of 0.150 M HCI is titrated with 0.075 M NaOH. What volume of NaOH is needed?a) 50 mLb) 20 mLc) 40 mLd) 26.66 mLarrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCI. What is the molarity of the HCI?a) 0.150 Mb) 0.079 Mc) 0.025 Md) 0.050 Marrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





