
Concept explainers
(a)
Interpretation:
The function of the sodium hydride has to be determined in the 1st step along with the
Concept introduction:
Aromaticity:
Aromaticity is a property of cyclic, planar structures with a ring of resonance bonds that gives increased stability compared to the other geometric or connective arrangements with the same set of atoms. Aromatic molecules are very stable and do not easily break up to take part in reactions.
There are some rules for a compound to be aromatic i.e. they have to be planar, cyclic and must contain
(a)
Explanation of Solution
In the given question the 1st step is,
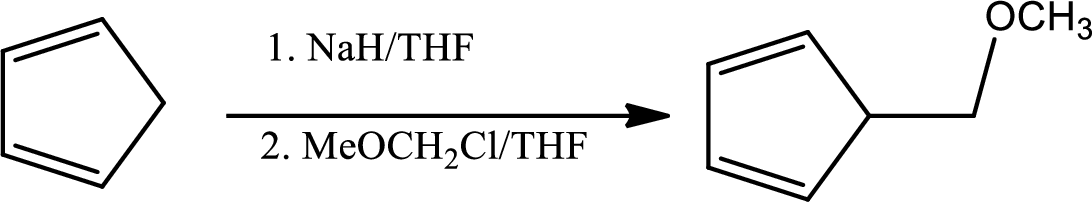
The mechanism as follows,
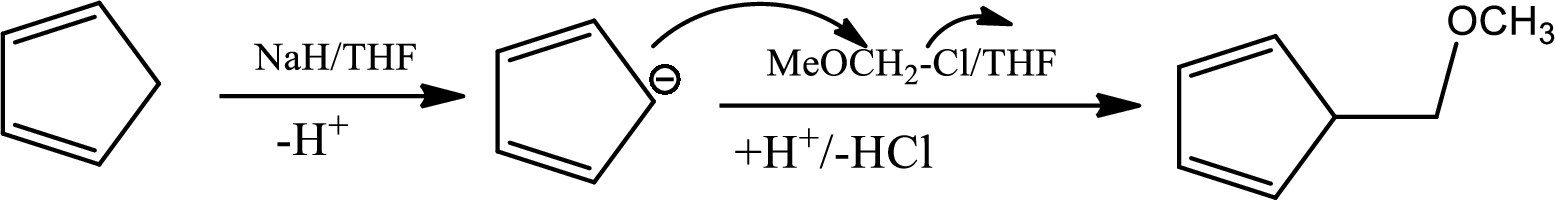
Here
The
(b)
Interpretation:
The reaction pathway by which B is converted to C that has to be identified.
Concept introduction:
Diels Alder reaction:
The Diels-Alder reaction is a
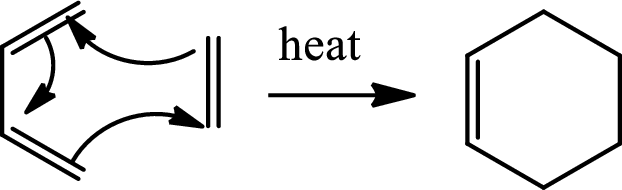
(b)
Explanation of Solution
According to the question,
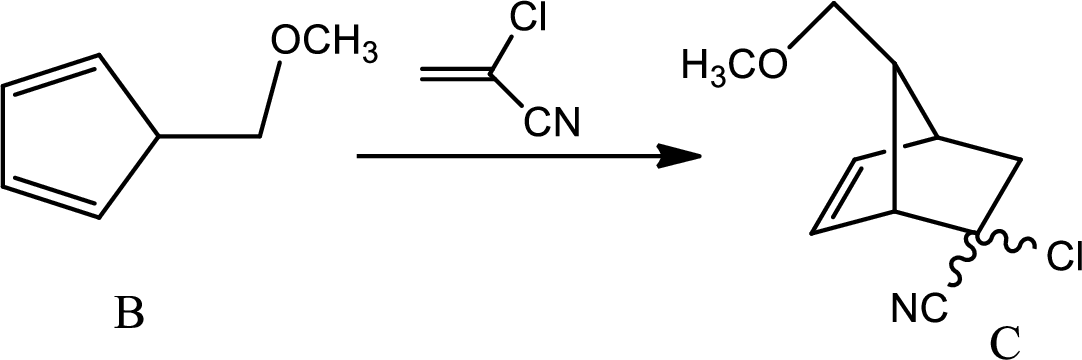
Here B is the diene and the dienophile consists of electron withdrawing groups.
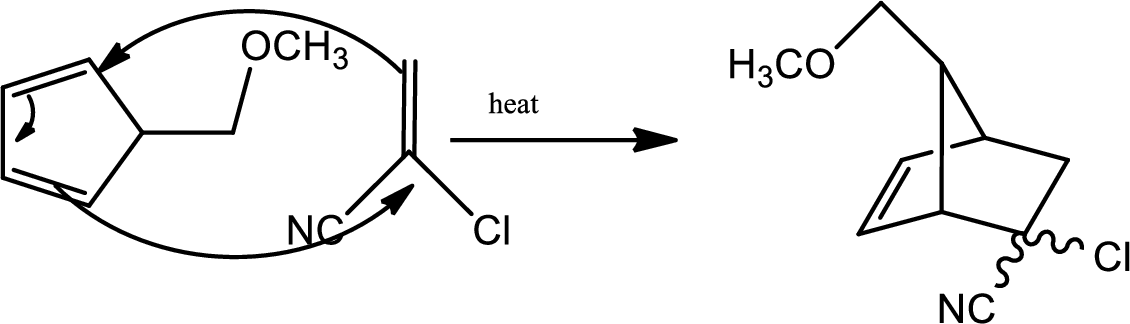
Thus via Diels Alder reaction the product is obtained.
(c)
Interpretation:
The role of the carbon dioxide added to the reaction mixture in step 2 of the conversion of (E) to (F) has to be determined.
Concept introduction:
Lactone formation:
Lactones are cyclic carboxylic esters that are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids which takes place spontaneously when th ering that is formed is five or six membered. The reaction pathway is as follows,
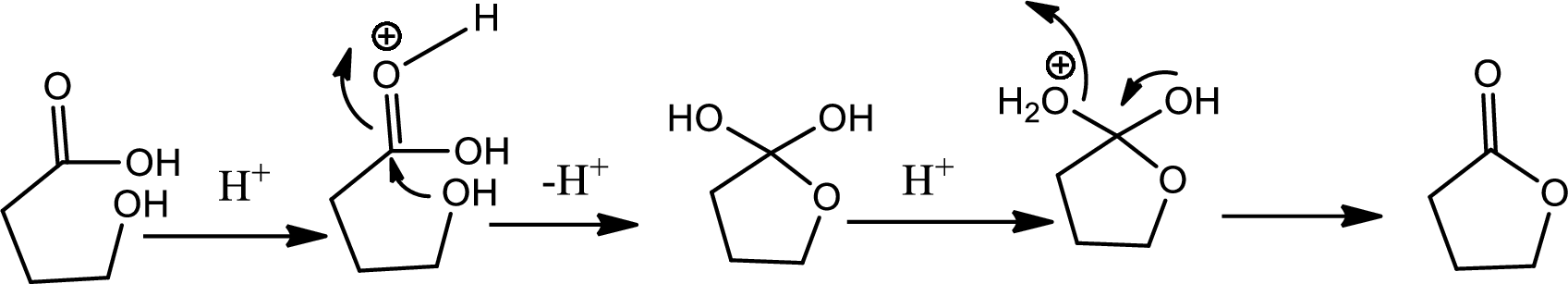
(c)
Explanation of Solution
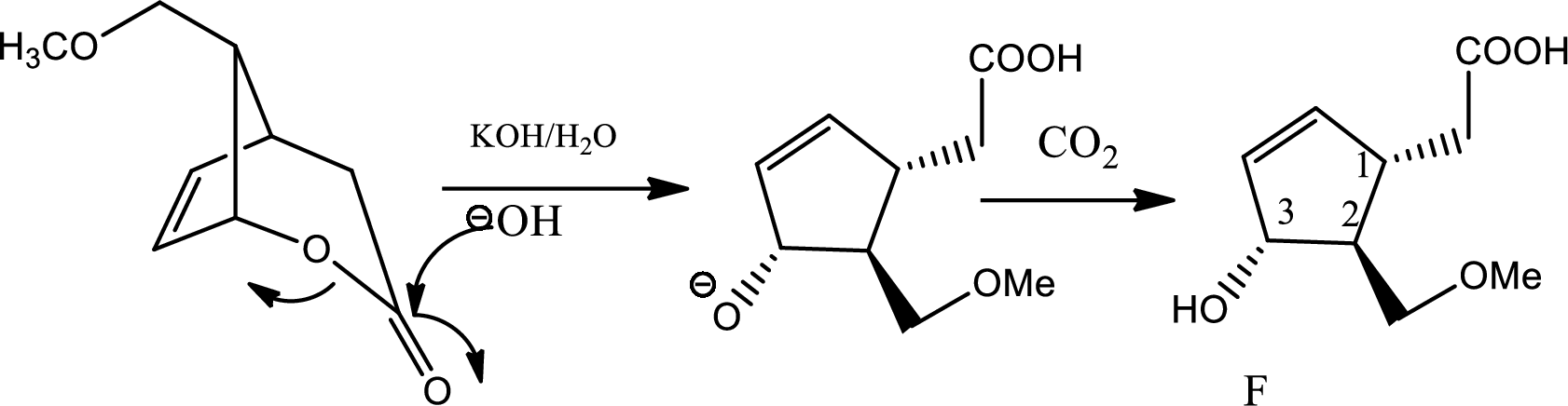
The reaction given is,
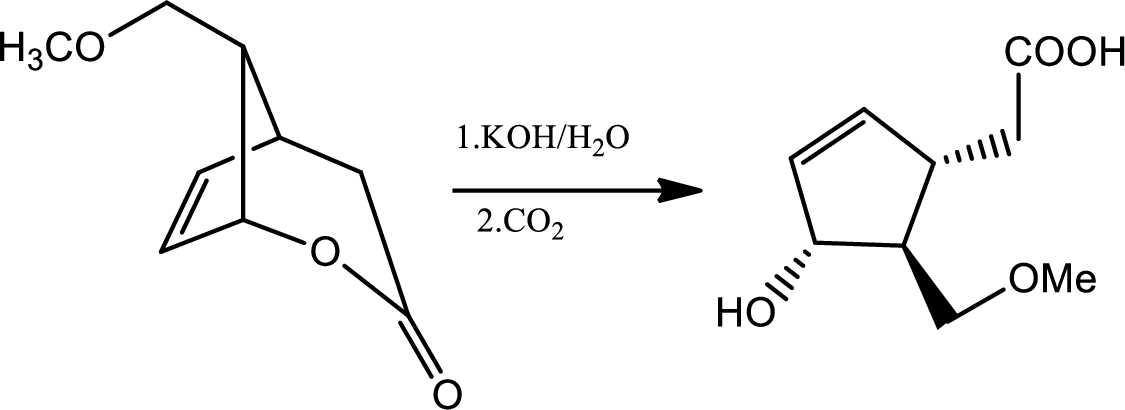
In this reaction in the presence of base lactone is hydrolised to give back acid and alcohol. Now if the amount of base is more in medium then again the backward reaction will be favoured and lactone will be formed again. So the reactant will be formed again. Hence to maintain the
Carbon dioxide reacts with water to give mild amount of carbonic acid that dissociates in water to a little extent to give less amount of proton which is sufficient to balance the
(d)
Interpretation:
A reaction pathway has to suggest for the conversion of (H) to (I) via radical mechanism.
Concept introduction:
Radical reaction:
A free radical reaction is a chemical reaction involving free radicals. Radical reaction contain three steps that are respectively chain initiation, chain propagation and chain termination.
(d)
Explanation of Solution
The reaction given,
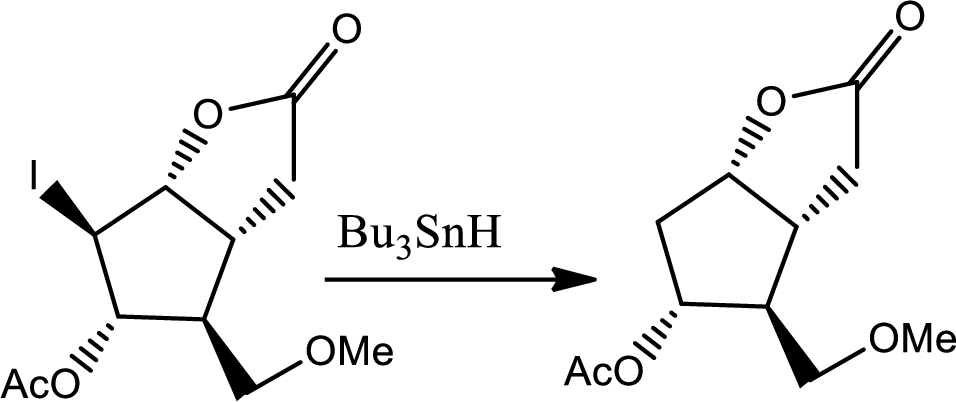
According to the question, the reaction proceeds via radical pathway and the 1st step involves a radical initiator to form
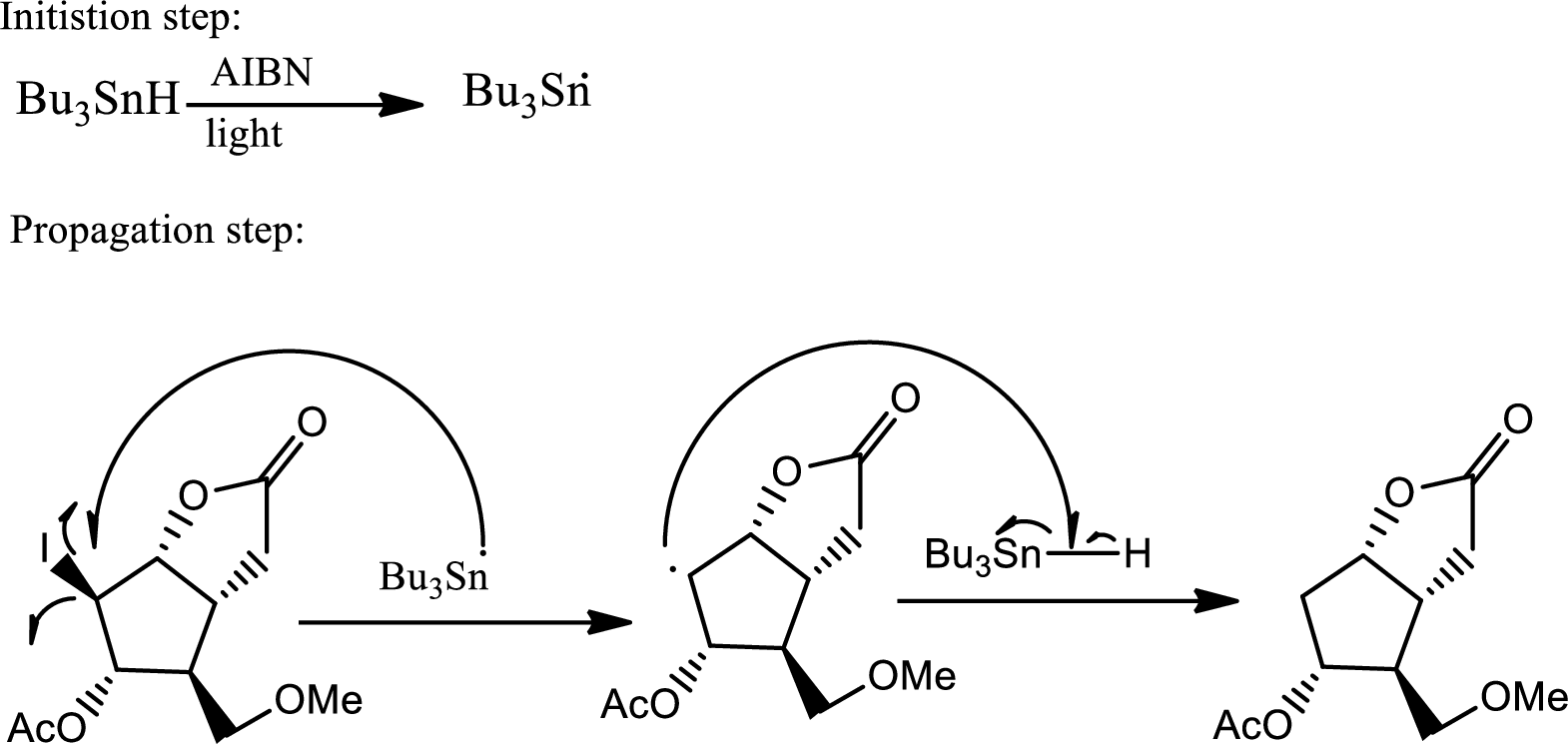
As a radical initiator AIBN is used. The reaction undergoes via two steps which are initiation and propagation respectively. Initiation step involves a radical initiator to form
(e)
Interpretation:
The steps in which the chiral centers of Corey lactone can be determined has to be shown with the mechanism.
Concept introduction:
Chiral centre:
Chiral centre is defined as an atom bonded to four different chemical species. It is a stereo centre that holds the atom in such way that the structure may not be superimposable to its mirror image. They give optical isomerism.
(e)
Explanation of Solution
In the question it is given that the Corey lactone has four chiral centers which are pointed below,
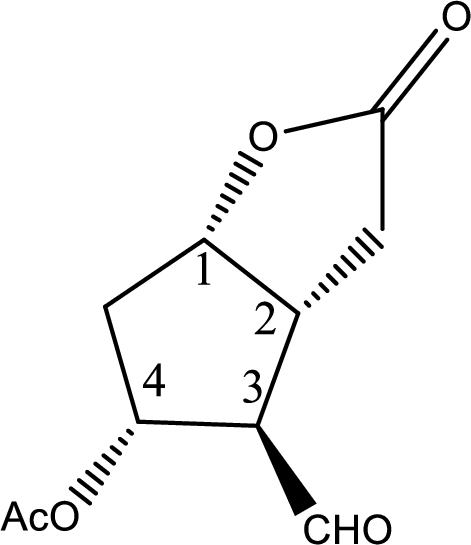
Now the steps where the chiral centers can be distinguished are given below,
In this step due to hydrolisation of lactone, ring opening occurs along with the formation of acid and alcohol.
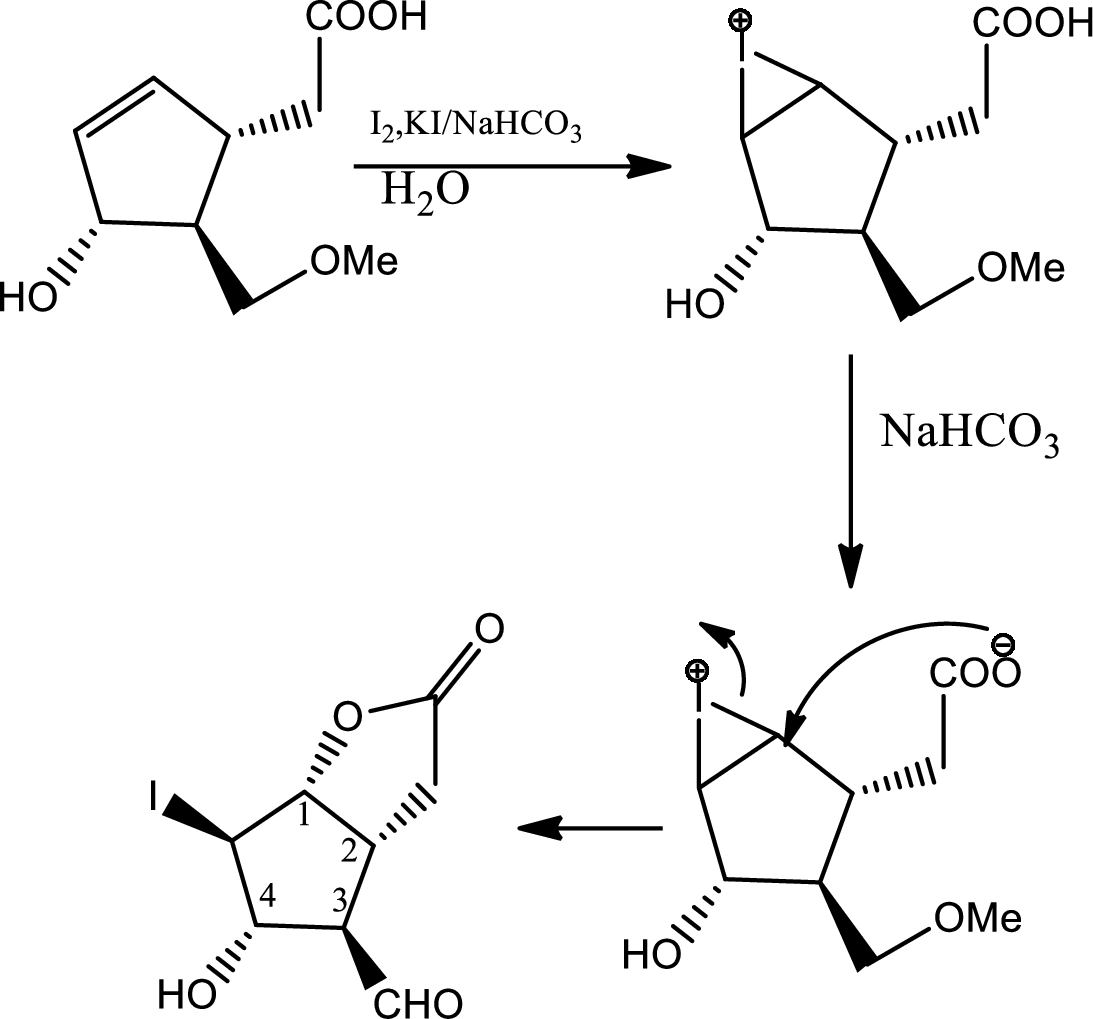
In this step normal
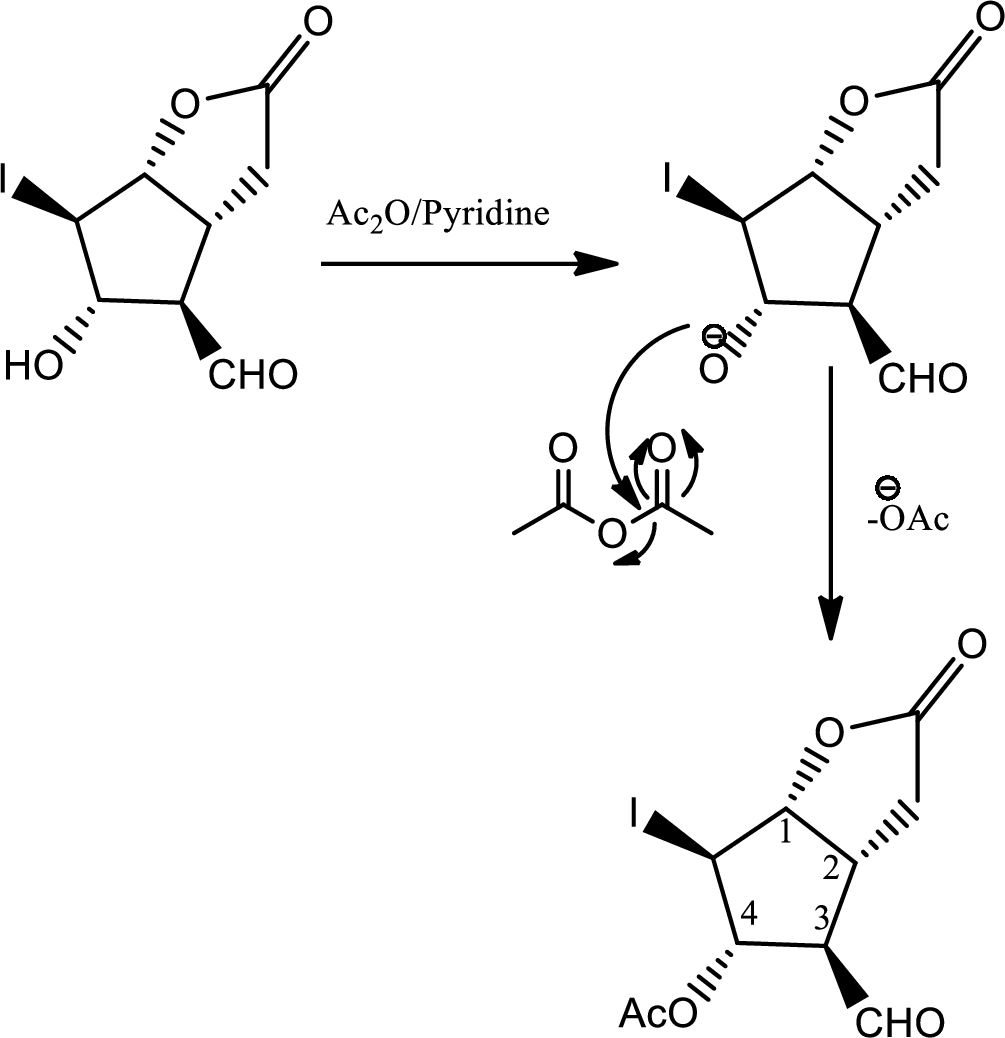
In this step normal acid base reaction occurs followed by normal
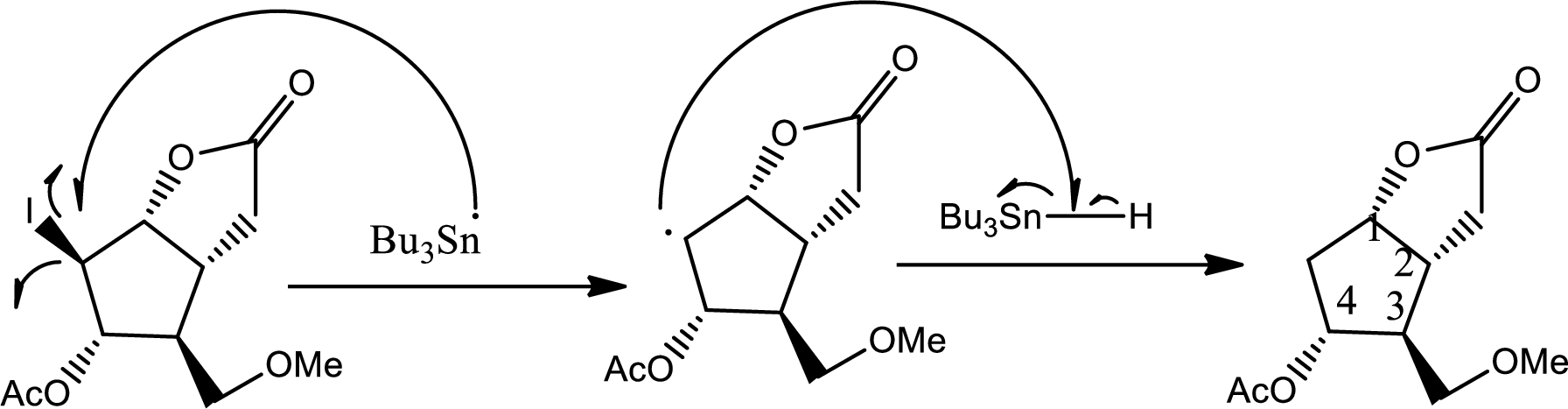
Via radical mechanism this step occurs to break the carbon-halogen bond.
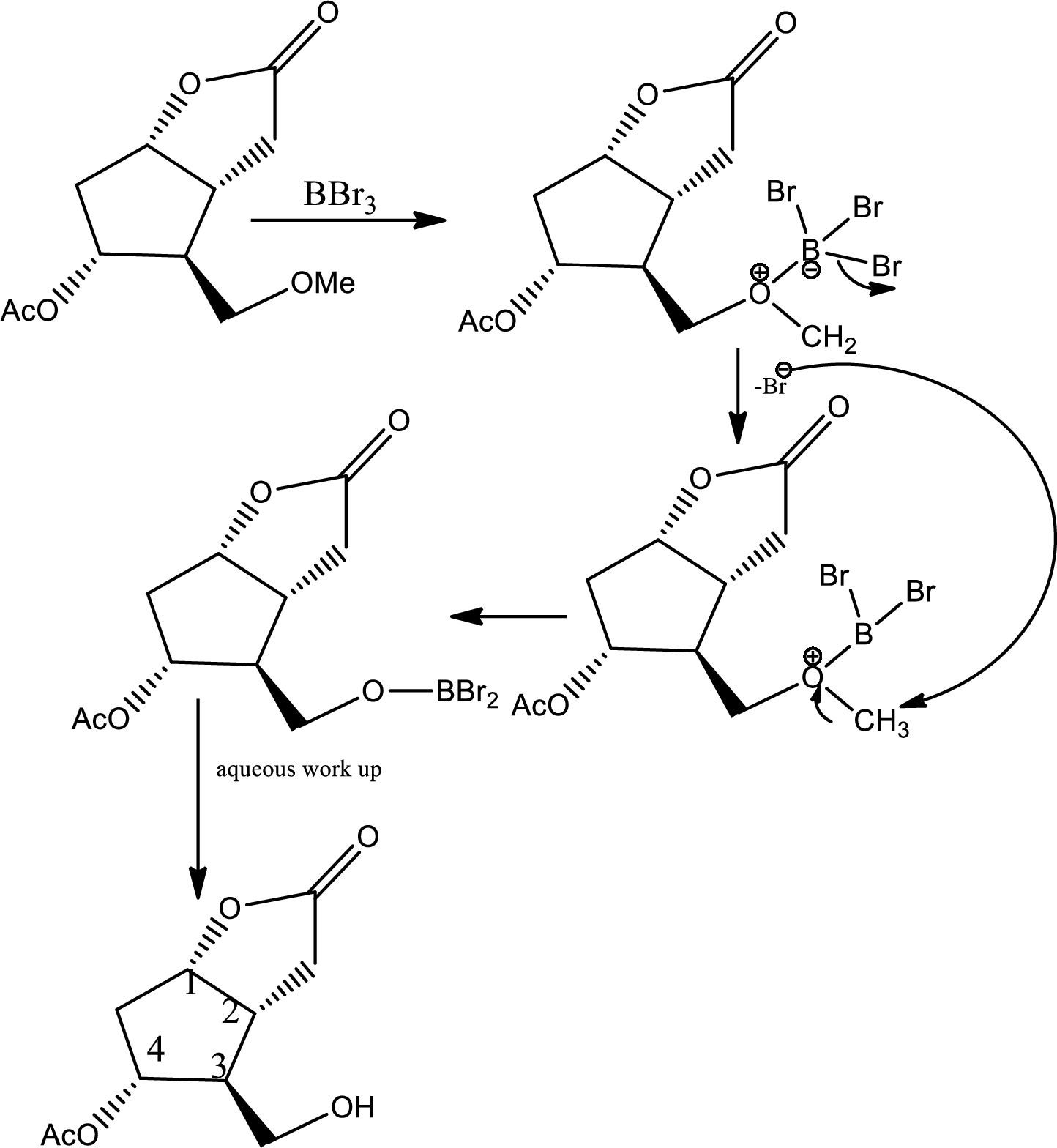
Here the ether is converted to alcohol.
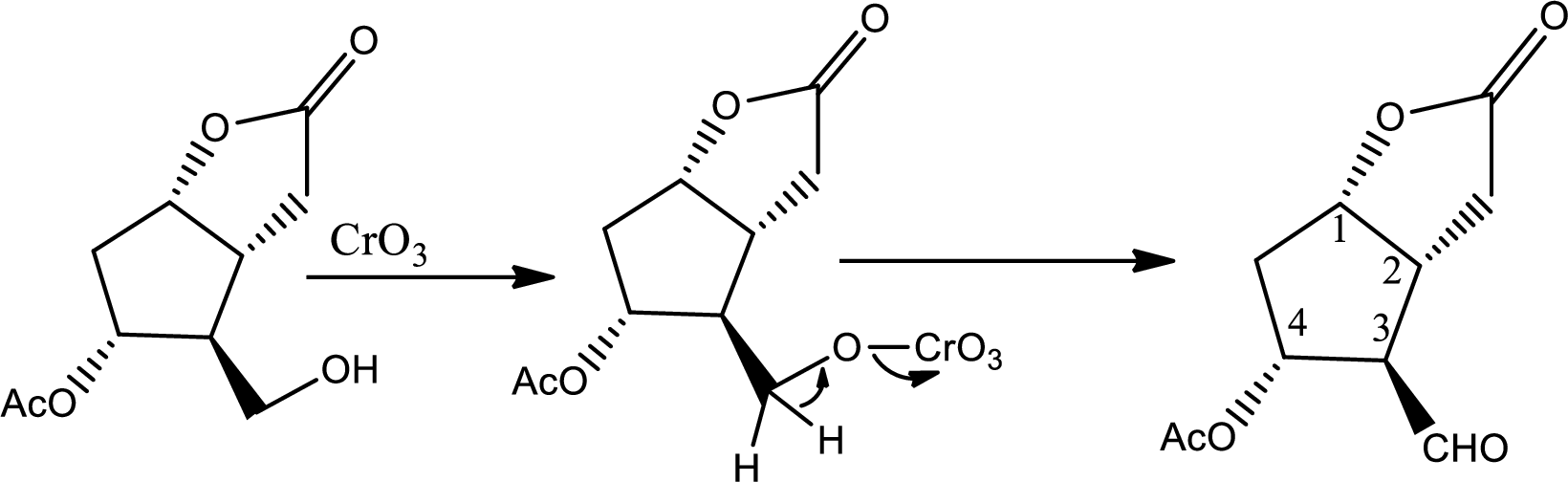
Here alcohol is oxidized to
In all these steps the chiral centres can be distinguished.
(f)
Interpretation:
The definition of resolution has to be given along with the rationale for using a chiral, enantiomerically pure
Concept introduction:
Resolution:
Chiral resolution in stereochemistry is a process for the seperation of racemic compounds into their enantiomers. Now racemic mixture is the mixture that has equal amounts of left and right handed enantiomers of the chiral molecule.
(f)
Explanation of Solution
According to the question the compound F formed is resolved by (+)-ephedrine. The structures of compound (F) and (-)-ephedrine are given below,
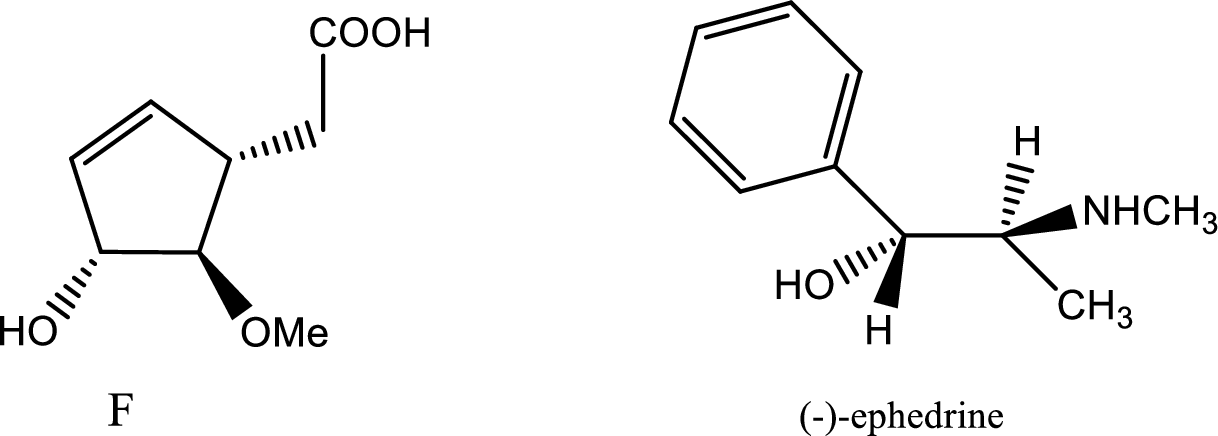
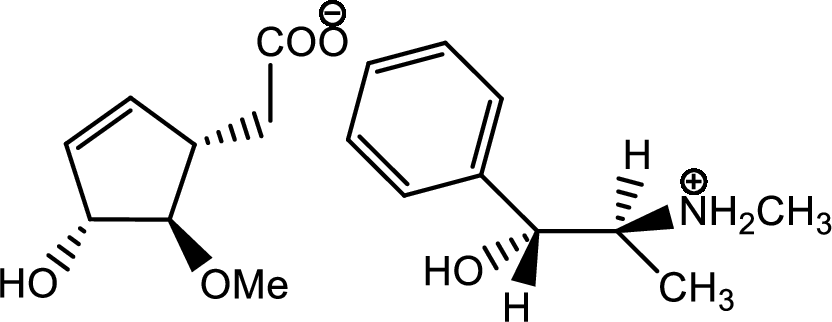
The process of racemisation follows acid base reaction between compound F and (+)-ephedrine. The two enantiomers can be converted to two diastereomeric salts those are [(+)(+)] and [(-)(+)]. Now being diastereomers they can be easily separated as diastereomers have different physical properties. Thus racemisation process works to separate two enantiomers.
One of the salts is shown below,
(g)
Interpretation:
The mechanism for the reaction pathway from D to E that undergoes via Baeyer-Villiger oxidation has to be shown.
Concept introduction:
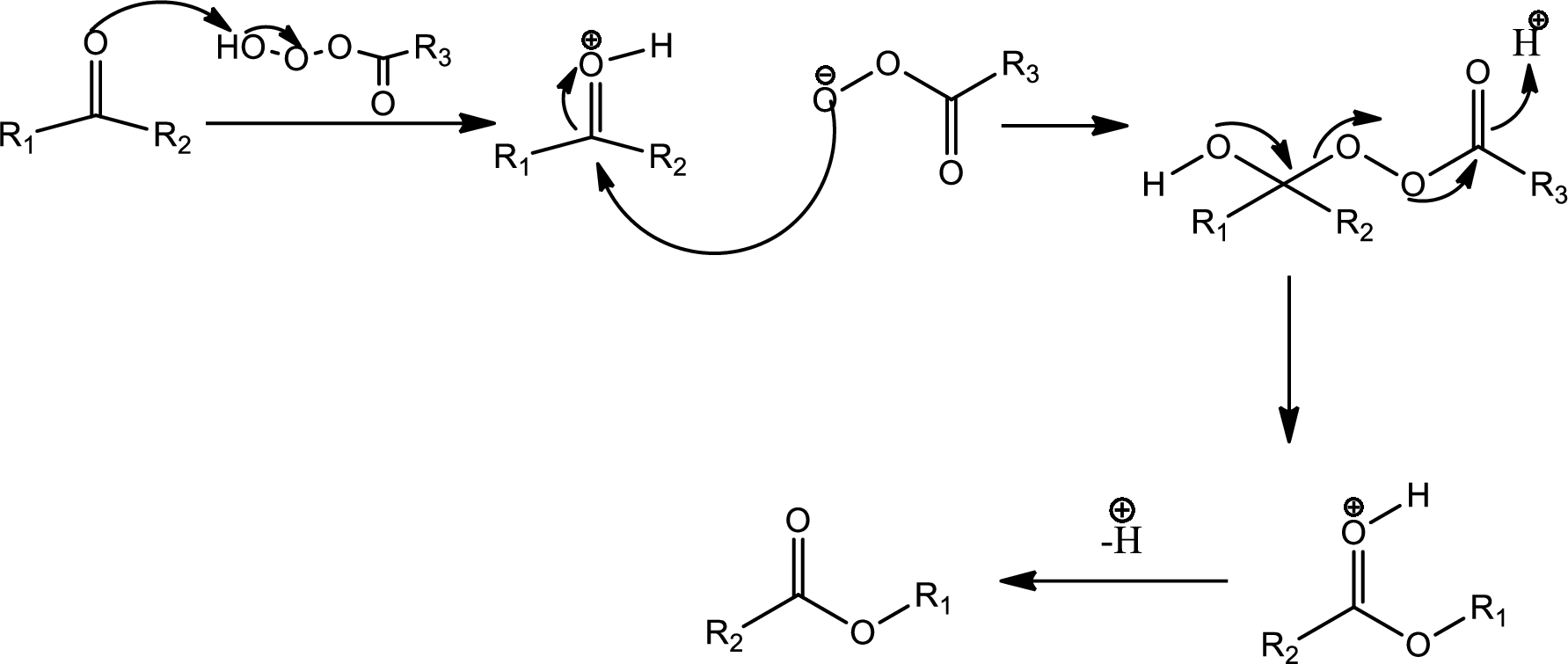
Baeyer-Villiger oxidation:
The Baeyer-Villiger oxidation is an organic rearrangement reaction that forms ester from a
(g)
Explanation of Solution
The reaction given in the question is,
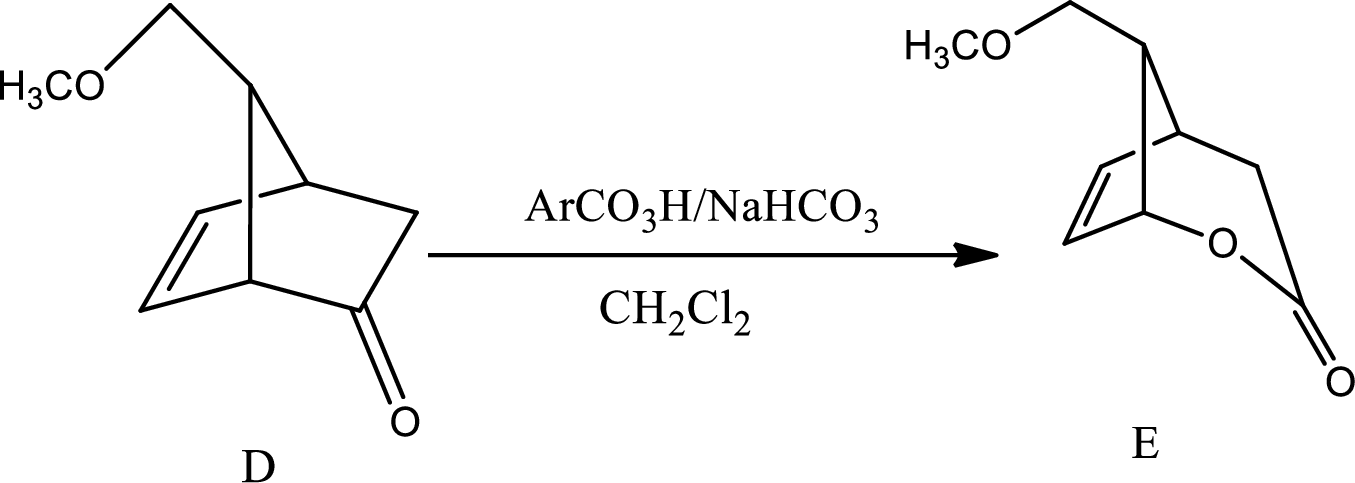
The mechanism is as follows,
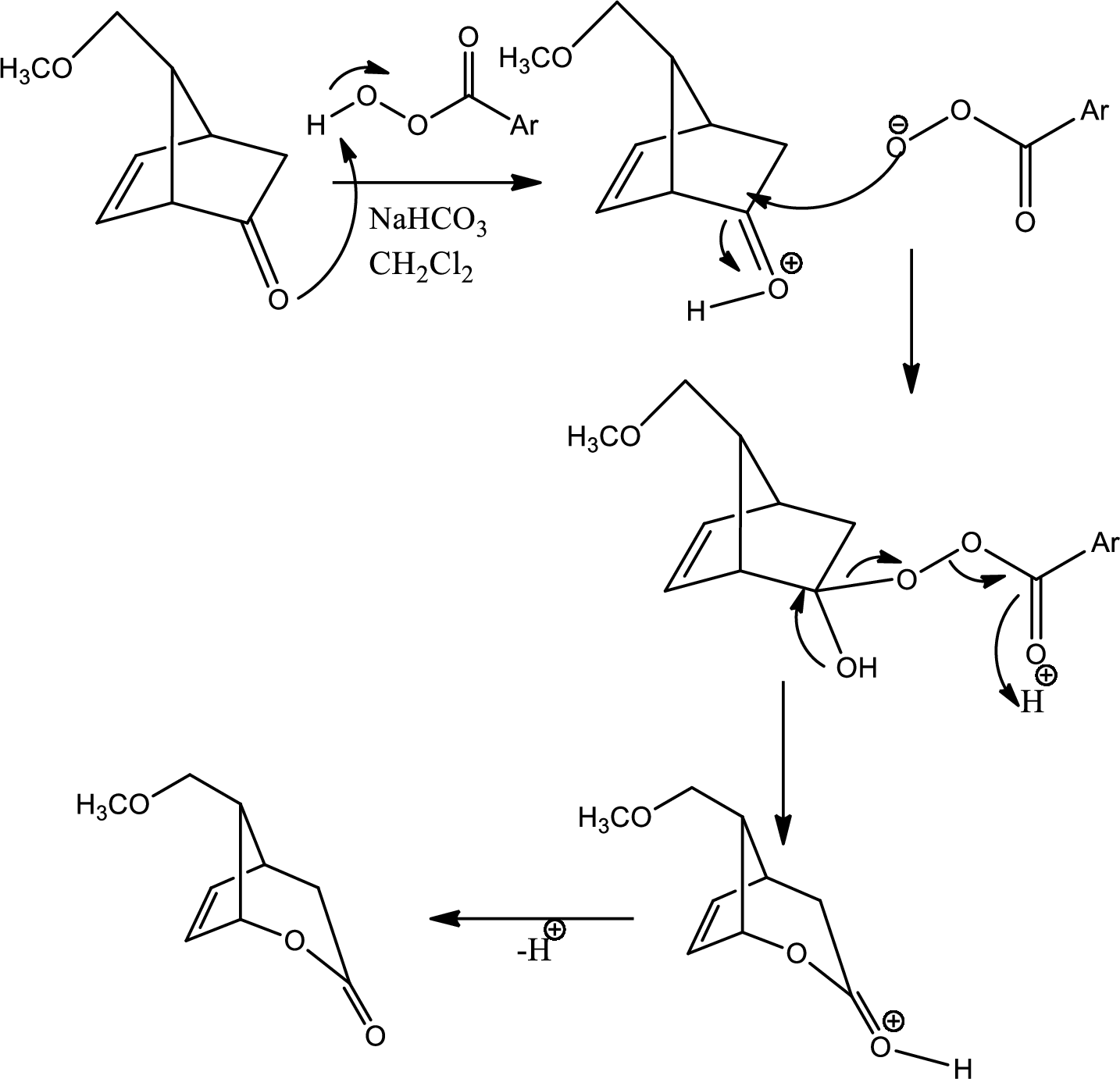
This is the pathway for D to E via Baeyer-Villiger oxidation. The process starts with acid base reaction followed by
Want to see more full solutions like this?
Chapter 24 Solutions
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
- Highlight the chirality (or stereogenic) center(s) in the given compound. A compound may have one or more stereogenic centers. OH OH OH OH OH OHarrow_forwardUsing wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration. NH H Br X टेarrow_forwardProvide photos of models of the following molecules. (Include a key for identification of the atoms) 1,2-dichloropropane 2,3,3-trimethylhexane 2-bromo-3-methybutanearrow_forward
- Please draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

