
ORGANIC CHEMISTRY-EBOOK>I<
9th Edition
ISBN: 9781305084414
Author: McMurry
Publisher: INTER CENG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.SE, Problem 30MP
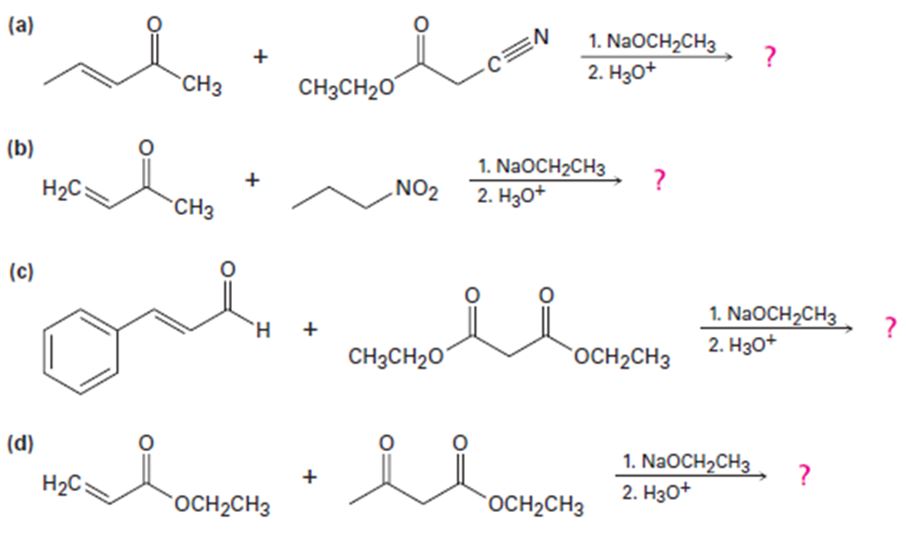
Predict the product(s) and provide the mechanism for each reaction below.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Alcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The
reaction mechanism typically involves a carbocation intermediate.
>
3rd attempt
3343
10
8
Draw arrows to show the reaction between the alkene and hydronium ion.
that
2nd attempt
Feedback
1st attempt
تعمال
Ju See Periodic Table See Hint
F
D
Ju See Periodic Table See Hint
Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product.
4th attempt
Π
Draw the simplified curved arrow mechanism
T
3rd attempt
Feedback
Ju
See Periodic Table See Hint
H
-H
H
-I
H
F
See Periodic Table See Hint
Select the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation
to show how it is converted to the final product.
4th attempt
Part 1 (0.5 point)
Select the correct reagent to accomplish the first step of this reaction.
Choose one:
OA Mg in ethanol (EtOH)
OB. 2 Li in THF
O C. Li in THF
D. Mg in THF
O E Mg in H2O
Part 2 (0.5 point)
Br
Part 1
Bri
Mg
CH
B
CH,
1 Draw intermediate here, but no arrows.
©
TE
See Periodic Table See Hint
See Hint
ין
H
Chapter 23 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Ch. 23.1 - Prob. 1PCh. 23.1 - Prob. 2PCh. 23.3 - Prob. 3PCh. 23.3 - Prob. 4PCh. 23.4 - Prob. 5PCh. 23.4 - 1-Butanol is prepared commercially by a route that...Ch. 23.4 - Prob. 7PCh. 23.5 - Prob. 8PCh. 23.6 - Prob. 9PCh. 23.6 - What product would you Expect to obtain from base...
Ch. 23.7 - Show the products you would expect to obtain by...Ch. 23.7 - Prob. 12PCh. 23.8 - What product would you expect from the following...Ch. 23.9 - What product would you expect From the following...Ch. 23.9 - Prob. 15PCh. 23.10 - Prob. 16PCh. 23.10 - Prob. 17PCh. 23.10 - Prob. 18PCh. 23.11 - Prob. 19PCh. 23.11 - Show how you might use an enamine reaction to...Ch. 23.12 - Prob. 21PCh. 23.12 - How would you prepare the following compound using...Ch. 23.SE - Prob. 23VCCh. 23.SE - Prob. 24VCCh. 23.SE - Prob. 25VCCh. 23.SE - The following molecule was formed by a Robinson...Ch. 23.SE - Prob. 27MPCh. 23.SE - Prob. 28MPCh. 23.SE - Predict the product(s) and provide the mechanism...Ch. 23.SE - Predict the product(s) and provide the mechanism...Ch. 23.SE - Predict the product(s) and provide the mechanism...Ch. 23.SE - Knoevenagel condensation is a reaction involving...Ch. 23.SE - Azlactones are important starting materials used...Ch. 23.SE - Prob. 34MPCh. 23.SE - Isoleucine, another of the twenty amino acids...Ch. 23.SE - The first step in the citric acid cycle of food...Ch. 23.SE - Prob. 37MPCh. 23.SE - The Knoevenagel reaction is a carbonyl...Ch. 23.SE - The Darzens reaction invoIves a two-step,...Ch. 23.SE - The following reaction involves a hydrolysis...Ch. 23.SE - Prob. 41MPCh. 23.SE - Prob. 42MPCh. 23.SE - Prob. 43MPCh. 23.SE - Propose a mechanism for the following...Ch. 23.SE - Prob. 45MPCh. 23.SE - Prob. 46MPCh. 23.SE - Prob. 47MPCh. 23.SE - Prob. 48APCh. 23.SE - Prob. 49APCh. 23.SE - Prob. 50APCh. 23.SE - Prob. 51APCh. 23.SE - Prob. 52APCh. 23.SE - Prob. 53APCh. 23.SE - Prob. 54APCh. 23.SE - Prob. 55APCh. 23.SE - Prob. 56APCh. 23.SE - Prob. 57APCh. 23.SE - Prob. 58APCh. 23.SE - In the mixed Claisen reaction of cyclopentanone...Ch. 23.SE - Ethyl dimethylacetoacetate reacts instantly at...Ch. 23.SE - Prob. 61APCh. 23.SE - Prob. 62APCh. 23.SE - The so-called Wieland-Miescher ketone is a...Ch. 23.SE - Prob. 64APCh. 23.SE - Prob. 65APCh. 23.SE - Prob. 66APCh. 23.SE - What condensation products would you expect to...Ch. 23.SE - The following reactions are unlikely to provide...Ch. 23.SE - Fill in the missing reagents a-h in the following...Ch. 23.SE - Prob. 70APCh. 23.SE - Prob. 71APCh. 23.SE - Prob. 72APCh. 23.SE - Prob. 73AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Select the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forward
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License