
Conceptual Integrated Science
3rd Edition
ISBN: 9780135197394
Author: Hewitt, Paul G., LYONS, Suzanne, (science Teacher), Suchocki, John, Yeh, Jennifer (jennifer Jean)
Publisher: PEARSON EDUCATION (COLLEGE)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 64TE
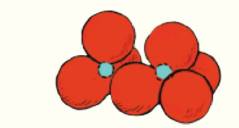
The drawing shows two silicate tetrahedra linked together. Redraw the figure. Label the oxygen atom that is shared between them.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2
3
Imagine you are out for a stroll on a sunny day when you encounter a lake. Unpolarized light from the sun is reflected off the lake into your eyes. However, you notice when you put on your vertically polarized sunglasses, the light reflected off the lake no longer reaches your eyes. What is the angle between the unpolarized light and the surface of the water, in degrees, measured from the horizontal? You may assume the index of refraction of air is nair=1 and the index of refraction of water is nwater=1.33 . Round your answer to three significant figures. Just enter the number, nothing else.
Chapter 23 Solutions
Conceptual Integrated Science
Ch. 23 - Diamond and graphite are minerals made of 100...Ch. 23 - Are plastic and nylon minerals?Ch. 23 - Describe the microscopic structure of a mineral.Ch. 23 - Is glass a mineral? Why or why not?Ch. 23 - What is the chemical formula for rubies? Why are...Ch. 23 - Identify six or more properties of minerals.Ch. 23 - How does the hardness of a mineral relate to its...Ch. 23 - Why is it rare to see large mineral crystals in...Ch. 23 - How many minerals are known to exist? What are the...Ch. 23 - Which is the largest group of minerals? What is...
Ch. 23 - Which are more common in Earths crust and mantle-...Ch. 23 - Identify seven classes of nonsilicate minerals.Ch. 23 - List four ways that minerals can form.Ch. 23 - A rock deep in Earth has a temperature higher than...Ch. 23 - When can the rock described in Exercise 14 melt to...Ch. 23 - Name an example of an evaporate mineral that you...Ch. 23 - Earths crust is made of rocks, so why dont we see...Ch. 23 - When cant we see the crystals in rocks?Ch. 23 - Do rocks have chemical formulas? Why or why not?Ch. 23 - What are the three major categories of rock?Ch. 23 - What is the difference between extrusive and...Ch. 23 - Why are most igneous rocks very hard?Ch. 23 - Prob. 23RCCCh. 23 - What is another name for intrusive igneous rocks?...Ch. 23 - Describe the process by which sedimentary rocks...Ch. 23 - Prob. 26RCCCh. 23 - How do clastic rocks differ from chemical rocks?Ch. 23 - What portion of the rock at Earths surface is...Ch. 23 - Heat and pressure can make one rock change, or...Ch. 23 - What kinds of rocks can undergo metamorphism?Ch. 23 - What are the stripes in foliated metamorphic rocks...Ch. 23 - The metamorphosis of shale into slate is an...Ch. 23 - Are rocks permanent features of Earth? Explain?Ch. 23 - How are all rocks related to one another?Ch. 23 - Five basic processes occur in the rock cycle. Name...Ch. 23 - What do all of the most common minerals in Earths...Ch. 23 - In what way is the silicate tetrahedron like a...Ch. 23 - What two elements make up the silicon tetrahedron?Ch. 23 - How are olivine, augite and feldspar alike? How...Ch. 23 - How is coal unlike other sedimentary rocks?Ch. 23 - Describe the formation of coal.Ch. 23 - What is coal made of?Ch. 23 - In what way are coal and petroleum alike?Ch. 23 - Prob. 44TISCh. 23 - Prob. 45TISCh. 23 - Prob. 46TISCh. 23 - Concrete is made from sand, gravel, and cement...Ch. 23 - a What is the average temperature of Earths...Ch. 23 - How does the atomic structure of glass differ from...Ch. 23 - The chemical formula for quartz is SiO2. Coesite...Ch. 23 - Toothbrushes and toothpastes usually consists of...Ch. 23 - Minerals in Earths crust generally do not contain...Ch. 23 - The chemical formula for quartz is SiO2. What is...Ch. 23 - Why do some high-quality drills have diamond tips?Ch. 23 - Why are quartz and diamond so much harder than...Ch. 23 - Name two properties of minerals that are based on...Ch. 23 - Prob. 59TECh. 23 - Why are the ferromagnesian silicates often dark,...Ch. 23 - What is more plentiful on Earththe group of...Ch. 23 - Olivine and augite are ferromagnesian silicates....Ch. 23 - Classify the following minerals as oxides,...Ch. 23 - The drawing shows two silicate tetrahedra linked...Ch. 23 - The chemical formula for the calcium-rich variety...Ch. 23 - Refer to figure 23.15b, which shows the structure...Ch. 23 - Why should mines be air-conditioned?Ch. 23 - One of your friends thinks that all mining should...Ch. 23 - Earths mineral resources are plentiful, but once...Ch. 23 - Prob. 70TECh. 23 - You have a tiny pile of quartz grains. You cover...Ch. 23 - Prob. 72TECh. 23 - Complete this sentence and explain your answer:...Ch. 23 - A geologist finds an igneous rock that has large...Ch. 23 - Why do rocks from slowly cooling magma have large...Ch. 23 - Prob. 76TECh. 23 - Why are intrusive igneous rocks coarse grained?...Ch. 23 - How is the magma that crystallizes to make...Ch. 23 - How can one magma body produce many different...Ch. 23 - Prob. 80TECh. 23 - Which of the three classes of rocks is formed at...Ch. 23 - Prob. 82TECh. 23 - Cycles in nature, such as the rock cycle, consist...Ch. 23 - Prob. 84TECh. 23 - Is the following rock a sedimentary rock, igneous...Ch. 23 - How is bituminous coal like coquina? How is...Ch. 23 - How is coal special among rocks?Ch. 23 - Prob. 88TECh. 23 - Prob. 89TECh. 23 - Why are metamorphic rocks formed underground?Ch. 23 - Prob. 91TECh. 23 - Metamorphism can be caused by pressure, heat, or...Ch. 23 - Cycles in nature, such as the rock cycle, consist...Ch. 23 - Identify a natural cycle other than the rock...Ch. 23 - If the 4.6-billion-year history of Earth were...Ch. 23 - A road cut reveals sedimentary strata. A low-lying...Ch. 23 - You read in the newspaper that a certain rock...Ch. 23 - The silicates are the largest mineral group...Ch. 23 - What physical change in metamorphic rock signals...Ch. 23 - Why do some minerals break down into cubes when...Ch. 23 - Which of these does not belong in your mineral...Ch. 23 - Which statement best describes how the majority of...Ch. 23 - Large crystals are usually associated with a...Ch. 23 - Why does the iridium layer in the rock record sug-...Ch. 23 - Prob. 8RATCh. 23 - Conglomerate is a sedimentary rock that consists...Ch. 23 - An igneous rock can be transformed into a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
Modified True/False 9. A giant bacterium that is large enough to be seen without a microscope is Selenomonas.
Microbiology with Diseases by Body System (5th Edition)
Why is petroleum jelly used in the hanging-drop procedure?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
1. Which is a function of the skeletal system? (a) support, (b) hematopoietic site, (c) storage, (d) providing ...
Anatomy & Physiology (6th Edition)
When a hydrochloric acid solution is combined with a potassium hydroxide solution, an acid-base reaction occurs...
Introductory Chemistry (6th Edition)
Draw the following orbitals: a. 3s orbital b. 4s orbital c. 3p orbital
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 20. Two small conducting spheres are placed on top of insulating pads. The 3.7 × 10-10 C sphere is fixed whie the 3.0 × 107 C sphere, initially at rest, is free to move. The mass of each sphere is 0.09 kg. If the spheres are initially 0.10 m apart, how fast will the sphere be moving when they are 1.5 m apart?arrow_forwardpls help on allarrow_forwardpls help on thesearrow_forward
- pls help on all asked questions kindlyarrow_forwardpls help on all asked questions kindlyarrow_forward19. Mount Everest, Earth's highest mountain above sea level, has a peak of 8849 m above sea level. Assume that sea level defines the height of Earth's surface. (re = 6.38 × 106 m, ME = 5.98 × 1024 kg, G = 6.67 × 10 -11 Nm²/kg²) a. Calculate the strength of Earth's gravitational field at a point at the peak of Mount Everest. b. What is the ratio of the strength of Earth's gravitational field at a point 644416m below the surface of the Earth to a point at the top of Mount Everest? C. A tourist watching the sunrise on top of Mount Everest observes a satellite orbiting Earth at an altitude 3580 km above his position. Determine the speed of the satellite.arrow_forward
- pls help on allarrow_forwardpls help on allarrow_forward6. As the distance between two charges decreases, the magnitude of the electric potential energy of the two-charge system: a) Always increases b) Always decreases c) Increases if the charges have the same sign, decreases if they have the opposite signs d) Increases if the charges have the opposite sign, decreases if they have the same sign 7. To analyze the motion of an elastic collision between two charged particles we use conservation of & a) Energy, Velocity b) Momentum, Force c) Mass, Momentum d) Energy, Momentum e) Kinetic Energy, Potential Energyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
 Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning


Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning


Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning
Newton's First Law of Motion: Mass and Inertia; Author: Professor Dave explains;https://www.youtube.com/watch?v=1XSyyjcEHo0;License: Standard YouTube License, CC-BY