
Organic Chemistry Third Edition + Electronic Solutions Manual And Study Guide
3rd Edition
ISBN: 9781119351610
Author: David Klein
Publisher: Wiley Plus
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 23, Problem 36PP
Interpretation Introduction
Interpretation:
The product for given reaction should be identified.
Concept Introduction:
Grubbs catalyst: This catalyst is used to achieve
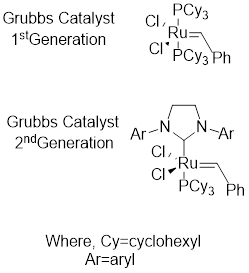
Alkene metathesis: It involves two stages first; the starting material in presence of catalyst forms two possible intermediates. Next the intermediates react with the starting material and results to form product.
Hydrogenation: The reaction in which proton is added over unsaturated given compound by using reagents. Hydrogenation is achieved by using
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4Help
Explain why the S-F bond strength is 367 kJ/mol in SF2 and 329 kJ/mol in SF6.
Would Si(CH3)3F react with AgCl? If so, write out the balanced chemical equation. If not,explain why no reaction would take place.
Chapter 23 Solutions
Organic Chemistry Third Edition + Electronic Solutions Manual And Study Guide
Ch. 23.1 - Identify which of the following reagents is...Ch. 23.2 - Prob. 2CCCh. 23.2 - Prob. 3CCCh. 23.2 - Prob. 4CCCh. 23.2 - Prob. 5CCCh. 23.2 - Prob. 6CCCh. 23.3 - Show how you would prepare 1-butylcyclopentene...Ch. 23.3 - Prob. 7PTSCh. 23.3 -
Using any two organohalides of your choice...Ch. 23.3 - Prob. 9ATS
Ch. 23.4 - Prob. 10CCCh. 23.4 - Prob. 11CCCh. 23.5 - Prob. 2LTSCh. 23.6 - Prob. 3LTSCh. 23.6 - Prob. 16PTSCh. 23.6 - Prob. 17PTSCh. 23.6 - Prob. 18ATSCh. 23.6 - Prob. 19ATSCh. 23.7 - Prob. 4LTSCh. 23.7 - Prob. 20PTSCh. 23.7 - Prob. 21PTSCh. 23.7 - Prob. 22ATSCh. 23.7 - Prob. 23ATSCh. 23.8 - Prob. 5LTSCh. 23.8 - Prob. 24PTSCh. 23.8 - Prob. 25ATSCh. 23.8 - Prob. 26ATSCh. 23.9 - Prob. 27CCCh. 23.9 - Prob. 28CCCh. 23.9 - Prob. 29CCCh. 23.9 - Prob. 6LTSCh. 23.9 - Prob. 30PTSCh. 23.9 - Prob. 31PTSCh. 23.9 - Prob. 32ATSCh. 23 - Prob. 33PPCh. 23 - Prob. 34PPCh. 23 - Prob. 35PPCh. 23 - Prob. 36PPCh. 23 - Prob. 37PPCh. 23 - Prob. 38PPCh. 23 - Prob. 39PPCh. 23 - Prob. 40PPCh. 23 - Prob. 41PPCh. 23 - Prob. 42PPCh. 23 - Prob. 43PPCh. 23 - Prob. 44PPCh. 23 - Prob. 45PPCh. 23 - Prob. 46PPCh. 23 - Using 1-pentene as your only source of carbon...Ch. 23 - Prob. 48PPCh. 23 - Prob. 49PPCh. 23 - Prob. 50PPCh. 23 - Prob. 51PPCh. 23 - Prob. 52PPCh. 23 - Prob. 53PPCh. 23 - Prob. 54PPCh. 23 - Prob. 55PPCh. 23 - Prob. 56PPCh. 23 - Prob. 57PPCh. 23 - Prob. 58PPCh. 23 - Prob. 59IPCh. 23 - Prob. 60IPCh. 23 - Prob. 61IPCh. 23 - Prob. 62IPCh. 23 - Prob. 64IPCh. 23 - Prob. 66IPCh. 23 - Prob. 68IPCh. 23 - Prob. 69IPCh. 23 - Prob. 70IPCh. 23 - Prob. 71CPCh. 23 - Prob. 72CPCh. 23 - Prob. 73CPCh. 23 - Prob. 74CPCh. 23 - Prob. 75CPCh. 23 - Prob. 76CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (2 Pts) Draw correct Lewis structures for two different molecules that have C3H6 as theirchemical formulaarrow_forwardSynthesize the following:arrow_forwardDid you report your data to the correct number of significant figures? Temperature of cold water (°C) 4.0 Temperature of hot water ("C) 87.0 Volume of cold water (mL) 94.0 Volume of hot water (mL) 78.0 Final temperature after mixing ("C) 41.0 Mass of cold water (g) 94.0 Mass of hot water (g) 78.0 Calorimeter constant (J/°C) 12.44 How to calculate the calorimeter constantarrow_forward
- please add appropriate arrows and tell me in detail where to add which or draw itarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY