
Concept explainers
(a)
Interpretation:
For the given complex the orbital splitting diagram has to be drawn using spectrochemial series.
Concept introduction:
The element in the periodic table and count its position in the respective transition series. These elements are in Periods 5 and 6, so the general configuration is
The spectrochemical series is
(a)
Explanation of Solution
Electron configuration of
Charge on
Electron configuration of
Six ligands indicate an octahedral arrangement. Using Hund’s rule, fill the lower energy
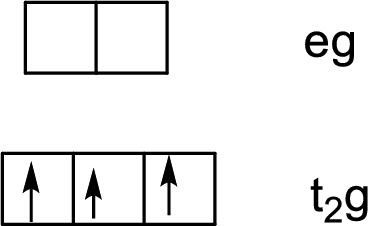
(b)
Interpretation:
For the given complex the orbital splitting diagram has to be drawn using spectrochemial series.
Concept introduction:
The element in the periodic table and count its position in the respective transition series. These elements are in Periods 5 and 6, so the general configuration is
The spectrochemical series is
(b)
Explanation of Solution
Electron configuration of
Charge on
Electron configuration of
Four ligands and a
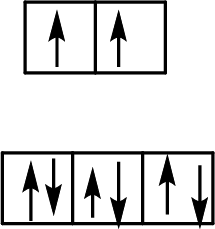
(c)
Interpretation:
For the given complex the orbital splitting diagram has to be drawn using spectrochemial series.
Concept introduction:
The element in the periodic table and count its position in the respective transition series. These elements are in Periods 5 and 6, so the general configuration is
The spectrochemical series is
(c)
Explanation of Solution
Electron configuration of
Charge on
Electron configuration of
Six ligands indicate an octahedral arrangement. Use Hund’s rule to fill the orbitals.
F– is a weak-field ligand, so the splitting energy, Δ, is not large enough to overcome the resistance to electron pairing. The electrons remain unpaired, and the complex is called high-spin.
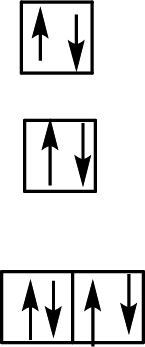
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: The Molecular Nature of Matter and Change - Standalone book
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forward
- Predict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





