
Concept explainers
(a)
Interpretation:
Molecular formula for the given set of compounds has to be written.
Concept Introduction:
Molecular formula of a compound represents the total number of atoms that is present in it. It does not give any other information about the
To Write: The molecular formula for the given ball-stick models.
(a)
Answer to Problem 23.23QP
The molecular formula for the first structure is
The molecular formula for the second structure is
The molecular formula for the third structure is
The molecular formula for the fourth structure is
Explanation of Solution
For First structure:
In the given ball-stick model, the black spheres are carbon atoms, light blue spheres are hydrogen atoms and dark blue spheres are nitrogen atoms. The total number of black spheres is 3, while that of light blue spheres is 9 and dark blue spheres is 1. Hence we arrive at the molecular formula of
For Second structure:
In the given ball-stick model, the black spheres are carbon atoms, light blue spheres are hydrogen atoms and red spheres are oxygen atoms. The total number of black spheres is 2, while that of light blue spheres is 4 and red spheres is 1. Hence we arrive at the molecular formula of
For Third structure:
In the given ball-stick model, the black spheres are carbon atoms, light blue spheres are hydrogen atoms and red spheres are oxygen atoms. The total number of black spheres is 3, while that of light blue spheres is 8 and red spheres is 1. Hence we arrive at the molecular formula of
For Fourth structure:
In the given ball-stick model, the black spheres are carbon atoms, light blue spheres are hydrogen atoms and red spheres are oxygen atoms. The total number of black spheres is 2, while that of light blue spheres is 4 and red spheres is 2. Hence we arrive at the molecular formula of
The molecular formula for the given ball-stick model compounds are written.
(b)
Interpretation:
Condensed structural formula for the given ball-stick model compounds has to be written.
Concept Introduction:
Condensed structural formula is representation of the organic compound. In this the lengthy carbon chain is shown only with the carbon atoms (along with the hydrogen) without any bonds. The additional branches are shown with explicit bonds.
To Write: The condensed structural formula for the given set of ball-stick model compounds.
(b)
Answer to Problem 23.23QP
The condensed structural formula of First structure is
The condensed structural formula for the Second structure is,

The condensed structural formula for the Third structure is,
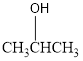
The condensed structural formula for the Fourth structure is,

Explanation of Solution
For First structure:
Condensed structural formula can be drawn considering the skeleton in the given ball-stick model. Nitrogen is the central atom which is bonded to three methyl groups. The single bonds between the nitrogen and methyl group are removed to obtain the condensed structural formula as shown below,

For Second structure:
Condensed structural formula can be drawn considering the carbon skeleton in the given ball-stick model. The carbon chain is drawn. One of the carbon atom is attached to an oxygen atom and a hydrogen atom. The single bonds are removed in the parent structure which results in the condensed structural formula as shown below,

For Third structure:
Condensed structural formula can be drawn considering the carbon skeleton in the given ball-stick model. The carbon chain is drawn. The carbon atom in the middle is attached to an hydroxy group. The single bonds are removed in the parent structure which results in the condensed structural formula as shown below,

For Fourth structure:
Condensed structural formula can be drawn considering the carbon skeleton in the given ball-stick model. The carbon chain is drawn. One of the carbon atom is attached to an hydroxy group and an oxygen atom. This means it is an

The condensed structural formula are written for the given ball-stick model representation of compounds.
(c)
Interpretation:
The functional group present in the given set of ball-stick models has to be identified.
Concept Introduction:
In an organic compound, the reactive portion is known as functional group. This undergoes reactions with other reagents and this does not depend upon how the rest of the compound is like. Few of the common functional groups are alcohol, ester, carboxylic acid,
To Identify: The functional group present in the given ball-stick models
(c)
Answer to Problem 23.23QP
The functional group present in First structure is identified as
The functional group present in Second structure is identified as aldehyde.
The functional group present in Third structure is identified as alcohol.
The functional group present in Fourth structure is identified as carboxylic acid.
Explanation of Solution
For First structure:
By looking at the formula of the given compound we can identify that a nitrogen is present in it. If nitrogen is present in a compound we can classify it as amine. In this case the nitrogen does not have any hydrogen atom bonded to it. Hence, it is a tertiary amine. The functional group is highlighted in red colour as shown.
For Second structure:
By looking at the formula of the given compound we can identify that a carbonyl group is present in it. The carbonyl group is attached to a hydrogen atom on one side and an alkyl group on other. Hence, this compound falls under the category of aldehyde.
For Third structure:
By looking at the formula of the given compound we can identify that a hydroxy group is present in it. The hydroxy group is attached to a carbon atom which bears only one hydrogen atoms. Hence, this compound falls under the category of secondary alcohol.
For Fourth structure:
By looking at the formula of the given compound we can identify that a carboxylic acid group is present in it. The carboxylic acid group is the functional group in this compound. Therefore, the compound falls under the category of carboxylic acid.
The functional groups present in the given set of ball-stick model compounds are identified.
Want to see more full solutions like this?
Chapter 23 Solutions
Student Solutions Manual for Ebbing/Gammon's General Chemistry
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardTake a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forward
- Show your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward= 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forward
- Predict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardThe problem will not be graded for correctness, but you have to get a reasonable answer something that is either correct or very closer to the correct answer. The instructor professor wants us to do something that shows the answer but everything does not have to be correct. Ideally, yes, it has to be correct. Give it your best shot.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





