
EBK COLLEGE PHYSICS, VOLUME 1
11th Edition
ISBN: 9781337514637
Author: Vuille
Publisher: CENGAGE LEARNING - CONSIGNMENT
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 15CQ
An object represented by a gray arrow, is placed in front of a plane mirror. Which of the diagram in Figure CQ23.15 best describes the image, represented by the pink arrow?
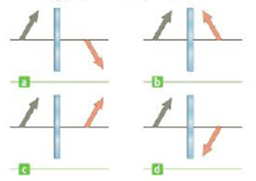
Figure CQ23.15
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
You are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?
For each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank you
A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).
Chapter 23 Solutions
EBK COLLEGE PHYSICS, VOLUME 1
Ch. 23.1 - In the overhead view if Figure 23.3, the image of...Ch. 23.3 - A person spearfishing from a boat sees a fish...Ch. 23.3 - True or False: (a) The image of an object placed...Ch. 23.5 - A clear plastic sandwich bag filled with water can...Ch. 23.5 - In Figure 23.25a, the blue object arrow is...Ch. 23.5 - An object is placed to the left of a converging...Ch. 23 - Tape a picture of yourself on a bathroom mirror....Ch. 23 - Prob. 2CQCh. 23 - The top row of Figure CQ23.3 shows three ray...Ch. 23 - Construct ray diagrams to determine whether each...
Ch. 23 - Construct ray diagrams to determine whether each...Ch. 23 - Prob. 6CQCh. 23 - Suppose you want to use a converging lens to...Ch. 23 - Lenses used in eyeglasses, whether converging or...Ch. 23 - In a Jules Verne novel, a piece of ice is shaped...Ch. 23 - If a cylinder of solid glass or clear plastic is...Ch. 23 - Prob. 11CQCh. 23 - Prob. 12CQCh. 23 - Why does the focal length of a mirror not depend...Ch. 23 - A person spear fishing from a boat sees a...Ch. 23 - An object represented by a gray arrow, is placed...Ch. 23 - (a) Does your bathroom mirror show you older or...Ch. 23 - Suppose you stand in front of a flat mirror and...Ch. 23 - Prob. 3PCh. 23 - In a church choir loft, two parallel walls are...Ch. 23 - A periscope (Fig. P23.5) is useful for viewing...Ch. 23 - A dentist uses a mirror to examine a tooth that is...Ch. 23 - A convex spherical mirror, whose focal length has...Ch. 23 - To fit a contact lens to a patient's eye, a...Ch. 23 - A virtual image is formed 20.0 cm from a concave...Ch. 23 - While looking at her image in a cosmetic minor,...Ch. 23 - Prob. 11PCh. 23 - A dedicated sports car enthusiast polishes the...Ch. 23 - A concave makeup mirror it designed to that a...Ch. 23 - A 1.80-m-tall person stands 9.00 m in front of a...Ch. 23 - A man standing 1.52 m in front of a shaving mirror...Ch. 23 - Prob. 16PCh. 23 - At an intersection of hospital hallways, a convex...Ch. 23 - The mirror of a solar cooker focuses the Suns rays...Ch. 23 - A spherical mirror is to be used to form an image,...Ch. 23 - Prob. 20PCh. 23 - A cubical block of ice 50.0 cm on an edge is...Ch. 23 - A goldfish is swimming inside a spherical bowl of...Ch. 23 - A paperweight is made of a solid hemisphere with...Ch. 23 - The top of a swimming pool is at ground level. If...Ch. 23 - A transparent sphere of unknown composition is...Ch. 23 - A man inside a spherical diving bell watches a...Ch. 23 - A jellyfish is floating in a water-filled aquarium...Ch. 23 - Figure P23.28 shows a curved surface separating a...Ch. 23 - A contact lens is made of plastic with an index of...Ch. 23 - A thin plastic lens with index of refraction n =...Ch. 23 - A converging lens has a local length of 10.0 cm....Ch. 23 - Prob. 32PCh. 23 - A diverging lens has a focal length of magnitude...Ch. 23 - A diverging lens has a focal length of 20.0 cm....Ch. 23 - Prob. 35PCh. 23 - The nickels image in Figure P23.36 has twice the...Ch. 23 - An object of height 8.00 cm it placed 25.0 cm to...Ch. 23 - An object is located 20.0 cm to the left of a...Ch. 23 - A converging lens is placed 30.0 cm to the right...Ch. 23 - (a) Use the thin-lens equation to derive an...Ch. 23 - Two converging lenses, each of focal length 15.0...Ch. 23 - A converging lens is placed at x = 0, a distance d...Ch. 23 - A 1.00-cm-high object is placed 4.00 cm to the...Ch. 23 - Two converging lenses having focal length of f1 =...Ch. 23 - Lens L1 in figure P23.45 has a focal length of...Ch. 23 - An object is placed 15.0 cm from a first...Ch. 23 - Prob. 47APCh. 23 - Prob. 48APCh. 23 - Prob. 49APCh. 23 - Prob. 50APCh. 23 - The lens and the mirror in figure P23.51 are...Ch. 23 - The object in Figure P23.52 is mid-way between the...Ch. 23 - Prob. 53APCh. 23 - Two rays travelling parallel to the principal axis...Ch. 23 - To work this problem, use the fact that the image...Ch. 23 - Consider two thin lenses, one of focal length f1...Ch. 23 - An object 2.00 cm high is placed 10.0 cm to the...Ch. 23 - Prob. 58APCh. 23 - Figure P23.59 shows a converging lens with radii...Ch. 23 - Prob. 60APCh. 23 - The lens-makers equation for a lens with index n1...Ch. 23 - An observer to the right of the mirror-lens...Ch. 23 - The lens-markers equation applies to a lens...Ch. 23 - Prob. 64APCh. 23 - A glass sphere (n = 1.50) with a radius of 15.0 cm...Ch. 23 - An object 10.0 cm tall is placed at the zero mark...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forward
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
- Discuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forward
- A 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forwardFor each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forwardWhen violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning
Convex and Concave Lenses; Author: Manocha Academy;https://www.youtube.com/watch?v=CJ6aB5ULqa0;License: Standard YouTube License, CC-BY