
(a)
Interpretation: The major products are to be found when the given compounds are treated with excess methyl iodide followed by aqueous silver oxide and heat.
Concept Introduction:
The reagents such as methyl iodide and silver oxide are used as the reaction conditions which are called Hofmann elimination. The
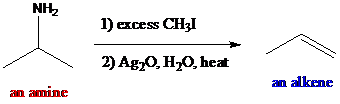
To find: The major product of cyclohexyl amine in Hofmann elimination
Draw and analyze the structure of cyclohexyl amine
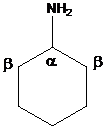
(b)
Interpretation: The major products are to be found when the given compounds are treated with excess methyl iodide followed by aqueous silver oxide and heat.
Concept Introduction:
The reagents such as methyl iodide and silver oxide are used as the reaction conditions which are called Hofmann elimination. The amines undergo exhaustive alkylation in the presence of excess methyl iodide producing quaternaryammonium salt which is excellent leaving group. After treatment with strong base, aqueous silver oxide and heat, the quaternary ammonium salt provides alkenes. Alkene formation involves in
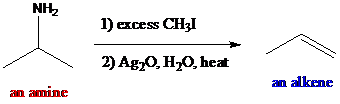
To find: The major product of (R)-3-methyl-2-butanamine in Hofmann elimination
Draw and analyze the structure of (R)-3-methyl-2-butanamine
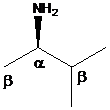
(c)
Interpretation: The major products are to be found when the given compounds are treated with excess methyl iodide followed by aqueous silver oxide and heat.
Concept Introduction:
The reagents such as methyl iodide and silver oxide are used as the reaction conditions which are called Hofmann elimination. The amines undergo exhaustive alkylation in the presence of excess methyl iodide producing quaternaryammonium salt which is excellent leaving group. After treatment with strong base, aqueous silver oxide and heat, the quaternary ammonium salt provides alkenes. Alkene formation involves in
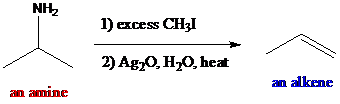
To find: The major product of N,N-dimethyl-1-phenylpropan-2-amine in Hofmann elimination
Draw and analyze the structure of N,N-dimethyl-1-phenylpropan-2-amine.
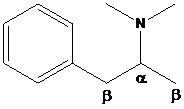
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry Third Edition + Electronic Solutions Manual And Study Guide
- What alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. and two equivalents of CH2=O draw structure ...arrow_forwardH-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forward
- Draw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





