
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22.8, Problem 23P
Which of the following compounds can serve as Michael acceptors?
a. 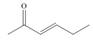 b.
b. 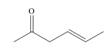 c.
c. 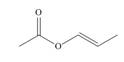 d.
d. 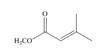
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction.
S₂O₃²⁻(aq) → S₄O₆²⁻(aq)
Q
Select to Edit
NH3
(CH3)2CHCI (1
equiv)
AICI 3
Select to Draw
cat. H2SO4
SO3 (1
equiv)
HO
SOCl2
pyridine
Select to Edit
>
Complete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction.
Zn(s) → Zn(OH)₄²⁻(aq)
Chapter 22 Solutions
Organic Chemistry (6th Edition)
Ch. 22.1 - Prob. 1PCh. 22.1 - Prob. 2PCh. 22.1 - Problem 24.3
What unsaturated carbonyl compound is...Ch. 22.1 - Prob. 4PCh. 22.1 - Prob. 5PCh. 22.2 - Prob. 6PCh. 22.2 - Problem 24.7
Draw the products formed in each...Ch. 22.4 - Prob. 15PCh. 22.4 - Prob. 16PCh. 22.5 - Problem 24.16
What ester is formed when each...
Ch. 22.6 - Prob. 18PCh. 22.6 - Prob. 19PCh. 22.6 -
Draw the products of each reaction.
a. b.
Ch. 22.6 - Problem 24.20
Two steps in a synthesis of the...Ch. 22.7 - Prob. 22PCh. 22.8 - Problem 24.22
Which of the following compounds can...Ch. 22.8 - Prob. 24PCh. 22.8 - Problem 24.24
What starting materials are needed...Ch. 22 - Prob. 29PCh. 22 - 24.29 What steps are needed to convert A to B?
Ch. 22 - Prob. 31PCh. 22 - 24.31 Draw the product formed in each directed...Ch. 22 - Prob. 33PCh. 22 - 24.33 What starting materials are needed to...Ch. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - 24.36 Identify the structures of C and D in the...Ch. 22 - Prob. 38PCh. 22 - Prob. 39PCh. 22 - 24.39 Draw the product formed from a Claisen...Ch. 22 - Prob. 41PCh. 22 - 24.41 Even though B contains three ester groups, a...Ch. 22 - Prob. 43PCh. 22 - Prob. 44PCh. 22 - 24.44 Vetivone is isolated from vetiver, a...Ch. 22 - Draw the product of each Robinson annulation from...Ch. 22 - Prob. 50PCh. 22 - Prob. 51PCh. 22 - 24.52 Draw a stepwise mechanism for the following...Ch. 22 - Prob. 53PCh. 22 - Prob. 54PCh. 22 - Prob. 55PCh. 22 - Prob. 56PCh. 22 - Prob. 57PCh. 22 - Prob. 58PCh. 22 - Prob. 59PCh. 22 - 24.62 Devise a synthesis of each compound from ,...Ch. 22 - Prob. 63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- b. ὋΗ CH3CH2OH H2SO4arrow_forwardFor the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forward
- ΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward:0: :0: Select to Add Arrows :0: (CH3)2NH :0: ■ Select to Add Arrows :0: :0: (CH3)2NH ■ Select to Add Arrowsarrow_forwardDraw the product of the following H action sequence. Ignore any inorganic byproducts formed. 1. (CH3CH2)2CuLi, THF 2. CH3Br Q Atoms, Bonds and Rings H Charges ㅁarrow_forward
- Please help me with this the problem is so confusingarrow_forward14 Question (1 point) Disiamylborane adds to a triple bond to give an alkenylborane. Upon oxidation with OH, H2O2, the alkenylborane will form an enol that tautomerizes to an aldehyde. In the first box below, draw the mechanism arrows for the reaction of disiamylborane with the alkyne, and in the last box draw the structure of the aldehyde. 4th attempt Feedback i > 3rd attempt OH, H2O2 i See Periodic Table See Hintarrow_forwardanswer with mechanisms and steps. handwritten please!arrow_forward
- Hello I need some help with Smartwork. For drawing structure B, I know the correct answer is CH₃B₂, but when I try to type it in, it keeps giving me CH₄BH₃ instead. Do you know how I should write it properly? Should I use a bond or something else?arrow_forwardTrue or false, chemistryarrow_forwardanswer thse questions with mechanisms and steps. handwritten please!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY