
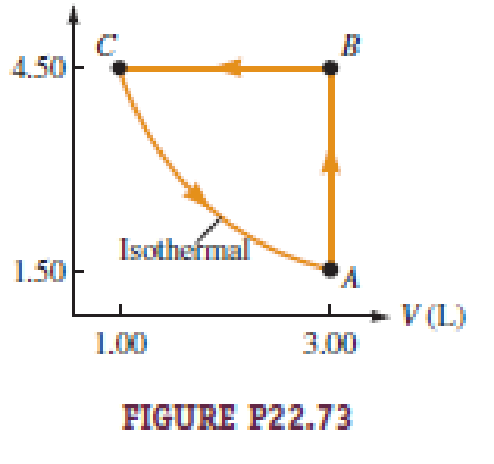
Figure P22.73 illustrates the cycle ABCA for a 2.00-mol sample of an ideal diatomic gas, where the process CA is a reversible isothermal expansion. What is a. the net work done by the gas during one cycle? b. How much energy is added to the gas by heat during one cycle? c. How much energy is exhausted from the gas by heat during one cycle? d. What is the efficiency of the cycle? e. What would be the efficiency of a Carnot engine operated between the temperatures at points A and B during each cycle?
(a)
The net work done by the gas during one cycle.
Answer to Problem 73PQ
The net work done by the gas during one cycle is
Explanation of Solution
The work done by the gas along AB is
Write the expression to calculate the work done by the gas along BC.
Here,
Write the expression to calculate the work done by the gas along CA.
Here,
Write the expression to calculate the total work done by the gas.
Here, W is the total work done by the gas.
Conclusion:
Substitute
Substitute
Substitute
Therefore, the net work done by the gas during one cycle is
(b)
The heat added in one cycle.
Answer to Problem 73PQ
The heat added in one cycle is
Explanation of Solution
For the diatomic gases, the specific heat capacity at constant volume is
The temperature at A and C of the gas is same.
Write the expression to calculate the temperature of the gas at A and C.
Here, T is the temperature of the gas at A and C, P is the pressure at A, V is the pressure at A n is the number of moles and R is the universal gas constant.
Write the expression to calculate the temperature at B.
Here,
Write the expression to calculate the energy added to the system.
Here, Q is the energy added to the system,
Substitute
Conclusion:
Substitute
Substitute
Substitute
Therefore, the heat added in one cycle is
(c)
The energy exhausted from the gas.
Answer to Problem 73PQ
The energy exhausted from the gas is
Explanation of Solution
For the diatomic gases, the specific heat capacity at constant pressure is
Write the expression to calculate the heat exhausted from the gas.
Here,
Substitute
Conclusion:
Substitute
Therefore, the energy exhausted from the gas is
(d)
The efficiency of the cycle.
Answer to Problem 73PQ
The efficiency of the cycle is
Explanation of Solution
Write the expression to calculate the efficiency of one cycle.
Here, e is the efficiency.
Conclusion:
Substitute
Therefore, the efficiency of the cycle is
(e)
The efficiency of the Carnot engine.
Answer to Problem 73PQ
The efficiency of the Carnot engine is
Explanation of Solution
Write the expression to calculate the efficiency of the Carnot engine.
Here,
Conclusion:
Substitute
Therefore, the efficiency of the Carnot engine is
Want to see more full solutions like this?
Chapter 22 Solutions
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
- The car goes from driving straight to spinning at 10.6 rev/min in 0.257 s with a radius of 12.2 m. The angular accleration is 4.28 rad/s^2. During this flip Barbie stays firmly seated in the car’s seat. Barbie has a mass of 58.0 kg, what is her normal force at the top of the loop?arrow_forwardConsider a hoop of radius R and mass M rolling without slipping. Which form of kinetic energy is larger, translational or rotational?arrow_forwardA roller-coaster vehicle has a mass of 571 kg when fully loaded with passengers (see figure). A) If the vehicle has a speed of 22.5 m/s at point A, what is the force of the track on the vehicle at this point? B) What is the maximum speed the vehicle can have at point B, in order for gravity to hold it on the track?arrow_forward
- This one wheeled motorcycle’s wheel maximum angular velocity was about 430 rev/min. Given that it’s radius was 0.920 m, what was the largest linear velocity of the monowheel?The monowheel could not accelerate fast or the rider would start spinning inside (this is called "gerbiling"). The maximum angular acceleration was 10.9 rad/s2. How long, in seconds, would it take it to hit maximum speed from rest?arrow_forwardIf points a and b are connected by a wire with negligible resistance, find the magnitude of the current in the 12.0 V battery.arrow_forwardConsider the two pucks shown in the figure. As they move towards each other, the momentum of each puck is equal in magnitude and opposite in direction. Given that v kinetic energy of the system is converted to internal energy? 30.0° 130.0 = green 11.0 m/s, and m blue is 25.0% greater than m 'green' what are the final speeds of each puck (in m/s), if 1½-½ t thearrow_forward
- Consider the blocks on the curved ramp as seen in the figure. The blocks have masses m₁ = 2.00 kg and m₂ = 3.60 kg, and are initially at rest. The blocks are allowed to slide down the ramp and they then undergo a head-on, elastic collision on the flat portion. Determine the heights (in m) to which m₁ and m2 rise on the curved portion of the ramp after the collision. Assume the ramp is frictionless, and h 4.40 m. m2 = m₁ m hm1 hm2 m iarrow_forwardA 3.04-kg steel ball strikes a massive wall at 10.0 m/s at an angle of 0 = 60.0° with the plane of the wall. It bounces off the wall with the same speed and angle (see the figure below). If the ball is in contact with the wall for 0.234 s, what is the average force exerted by the wall on the ball? magnitude direction ---Select--- ✓ N xarrow_forwardYou are in the early stages of an internship at NASA. Your supervisor has asked you to analyze emergency procedures for extravehicular activity (EVA), when the astronauts leave the International Space Station (ISS) to do repairs to its exterior or perform other tasks. In particular, the scenario you are studying is a failure of the manned-maneuvering unit (MMU), which is a nitrogen-propelled backpack that attaches to the astronaut's primary life support system (PLSS). In this scenario, the astronaut is floating directly away from the ISS and cannot use the failed MMU to get back. Therefore, the emergency plan is to take off the MMU and throw it in a direction directly away from the ISS, an action that will hopefully cause the astronaut to reverse direction and float back to the station. You have the following mass data provided to you: astronaut: 78.1 kg, spacesuit: 36.8 kg, MMU: 115 kg, PLSS: 145 kg. Based on tests performed by astronauts floating "weightless" inside the ISS, the most…arrow_forward
- Three carts of masses m₁ = 4.50 kg, m₂ = 10.50 kg, and m3 = 3.00 kg move on a frictionless, horizontal track with speeds of V1 v1 13 m 12 mq m3 (a) Find the final velocity of the train of three carts. magnitude direction m/s |---Select--- ☑ (b) Does your answer require that all the carts collide and stick together at the same moment? ○ Yes Ο Νο = 6.00 m/s to the right, v₂ = 3.00 m/s to the right, and V3 = 6.00 m/s to the left, as shown below. Velcro couplers make the carts stick together after colliding.arrow_forwardA girl launches a toy rocket from the ground. The engine experiences an average thrust of 5.26 N. The mass of the engine plus fuel before liftoff is 25.4 g, which includes fuel mass of 12.7 g. The engine fires for a total of 1.90 s. (Assume all the fuel is consumed.) (a) Calculate the average exhaust speed of the engine (in m/s). m/s (b) This engine is positioned in a rocket body of mass 70.0 g. What is the magnitude of the final velocity of the rocket (in m/s) if it were to be fired from rest in outer space with the same amount of fuel? Assume the fuel burns at a constant rate. m/sarrow_forwardTwo objects of masses m₁ 0.48 kg and m₂ = 0.86 kg are placed on a horizontal frictionless surface and a compressed spring of force constant k 260 N/m is placed between them as in figure (a). Neglect the mass of the spring. The spring is not attached to either object and is compressed a distance of 9.4 cm. If the objects are released from rest, find the final velocity of each object as shown in figure (b). (Let the positive direction be to the right. Indicate the direction with the sign of your answer.) m/s V1 V2= m1 m/s k m2 a す。 k m2 m1 barrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





