
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
8th Edition
ISBN: 9780100547964
Author: Hurley
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 22, Problem 46QAP
Interpretation Introduction
(a)
Interpretation:
The chiral carbon(s), if any, in the following molecule should be located.
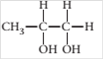
Concept introduction:
When a carbon atom in a molecule is bonded to four different groups or atoms then it is said to be chiral carbon.
Interpretation Introduction
(b)
Interpretation:
The chiral carbon(s), if any, in the following molecule should be located.
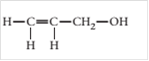
Concept introduction:
When a carbon atom in a molecule is bonded to four different groups or atoms then it is said to be chiral carbon.
Interpretation Introduction
(c)
Interpretation:
The chiral carbon(s), if any, in the following molecule should be located.
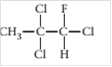
Concept introduction:
When a carbon atom in a molecule is bonded to four different groups or atoms then it is said to be chiral carbon.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Name the following hydrocarbons. (9 marks)
a)
HHHHHHHH
H-C-C-
H-O-S
b)
HCEC-CH3
H H
H H
H
d)
c)
H
C=C-
H
H
H
e)
CH3
CH3 CH2CH=CH-CH=CHCH3
HHHH
H-C-C-C-C-H
H
HH H
f)
large
CH2CH3
pola
H3C
section
lovels
tower,
able
ocart
firs g)
Tower
H3C-CH2
then in
H3C-CH-CH-CH3
enblbano bne noitsidab
Copyright © 2008. Durham Continuing Education
CH3
Name the molecules & Identify any chiral center
CH3CH2CH2CHCH₂CH₂CH₂CH₂
OH
CH₂CHCH2CH3
Br
CH3
CH3CHCH2CHCH2CH3
CH3
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).
Chapter 22 Solutions
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
Ch. 22 - Classify each of the following hydrocarbons as...Ch. 22 - Classify each of the following hydrocarbons as...Ch. 22 - Write the formula for (a) an alkene with two...Ch. 22 - Write the formula for (a) an alkyne with 16...Ch. 22 - Name the following alkanes.Ch. 22 - Name the following alkanes.Ch. 22 - Prob. 7QAPCh. 22 - Prob. 8QAPCh. 22 - The following names are incorrect; draw a...Ch. 22 - Prob. 10QAP
Ch. 22 - Prob. 11QAPCh. 22 - Prob. 12QAPCh. 22 - Prob. 13QAPCh. 22 - Name the following compounds as derivatives of...Ch. 22 - Prob. 15QAPCh. 22 - Prob. 16QAPCh. 22 - Prob. 17QAPCh. 22 - Prob. 18QAPCh. 22 - Prob. 19QAPCh. 22 - Prob. 20QAPCh. 22 - Prob. 21QAPCh. 22 - Prob. 22QAPCh. 22 - Prob. 23QAPCh. 22 - Arrange these compounds in order of increasing...Ch. 22 - The Kbfor ethylamine (CH3CH2NH2) is 4.3104 . What...Ch. 22 - When aniline, C6H5NH2(Kb=7.41010) , reacts with a...Ch. 22 - When ethylamine, a weak base (Kb=4.3104) , reacts...Ch. 22 - When the conjugate acid of aniline, C6H5NH3+,...Ch. 22 - Draw the structural isomers of the alkane C6H14.Ch. 22 - Prob. 30QAPCh. 22 - Prob. 31QAPCh. 22 - Draw the structural isomers of C3H6Cl2 in which...Ch. 22 - Prob. 33QAPCh. 22 - Prob. 34QAPCh. 22 - Prob. 35QAPCh. 22 - Prob. 36QAPCh. 22 - Draw structures for all the alcohols with...Ch. 22 - Prob. 38QAPCh. 22 - Prob. 39QAPCh. 22 - Prob. 40QAPCh. 22 - Maleic acid and fumaric acid are the cis- and...Ch. 22 - Prob. 42QAPCh. 22 - Which of the following can show optical isomerism?...Ch. 22 - Prob. 44QAPCh. 22 - Prob. 45QAPCh. 22 - Prob. 46QAPCh. 22 - Prob. 47QAPCh. 22 - Prob. 48QAPCh. 22 - Prob. 49QAPCh. 22 - Prob. 50QAPCh. 22 - Prob. 51QAPCh. 22 - Prob. 52QAPCh. 22 - Calculate [H+] and the pH of a 0.10 M solution of...Ch. 22 - Prob. 54QAPCh. 22 - The general formula of an alkane is CnH2n+2 . What...Ch. 22 - Prob. 56QAPCh. 22 - Prob. 57QAPCh. 22 - Prob. 58QAPCh. 22 - Prob. 59QAPCh. 22 - Write an equation for the reaction of chloroacetic...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardWhat is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forwardLook at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY