
Interpretation:
Based on the given information, the structures of compounds A-F are to be proposed.
Concept introduction:
A carbohydrate is a
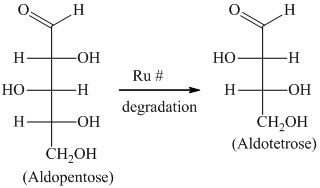
The Ruff degradation reaction is used for decreasing the number of carbon atoms of the respective aldoses, thereby shortening the carbon chain of the compound. It works in two steps:
Using bromine water to oxidize an aldose to the respective aldonic acid.
Using
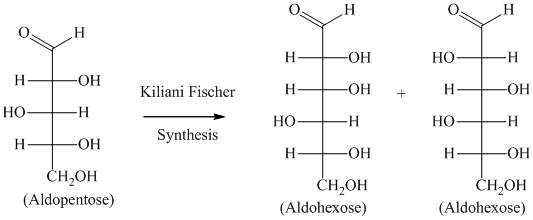
The Kiliani-Fischer reaction is used for producing epimers of higher aldoses from a lower aldose.
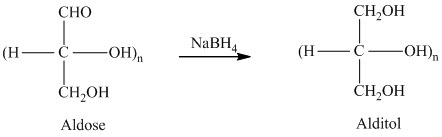
Alditols are sugar alcohols formed by the addition of hydrogen to the monosaccharide. Reduction of monosaccharides (carbohydrates containing single units of sugar molecules) results in the formation of alditols.
The molecules that are non-superimposable or not identical with their mirror images are known as chiral molecules.
A pair of two mirror images that are non-identical are known as enantiomers, which are optically active.
The stereoisomers that are non-superimposable on each other and not mirror images of each other are known as diastereomers.
The achiral compounds in which plane of symmetry is present internally and consists of chiral centres are known as meso compounds, but they are optically inactive.
Compounds that have a plane of symmetry tend to exist in meso forms. A meso form arises when the two stereoisomers produce superimposable images, and hence, compounds having meso forms are optically inactive.
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
ORGANIC CHEM. VOL.1+2-W/WILEYPLUS
- H-Br Energy 1) Draw the step-by-step mechanism by which 3-methylbut-1-ene is converted into 2-bromo-2-methylbutane. 2) Sketch a reaction coordinate diagram that shows how the internal energy (Y- axis) of the reacting species change from reactants to intermediate(s) to product. Brarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 H-CI CH2Cl2 CIarrow_forwardDraw the products of the stronger acid protonating the other reactant. དའི་སྐད”“ H3C OH H3C CH CH3 KEq Product acid Product basearrow_forward
- Draw the products of the stronger acid protonating the other reactant. H3C NH2 NH2 KEq H3C-CH₂ 1. Product acid Product basearrow_forwardWhat alkene or alkyne yields the following products after oxidative cleavage with ozone? Click the "draw structure" button to launch the drawing utility. draw structure ... andarrow_forwardDraw the products of the stronger acid protonating the other reactant. H3C-C=C-4 NH2 KEq CH H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). C5H10 Br H-Br CH2Cl2 + enant.arrow_forwardDraw the products of the stronger acid protonating the other reactant. KEq H₂C-O-H H3C OH Product acid Product basearrow_forwardDraw the products of the stronger acid protonating the other reactant. OH KEq CH H3C H3C `CH3 Product acid Product basearrow_forward
- 2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). Ph H-I CH2Cl2arrow_forward3 attempts left Check my work Draw the products formed in the following oxidative cleavage. [1] 03 [2] H₂O draw structure ... lower mass product draw structure ... higher mass productarrow_forward2. Draw the missing structure(s) in each of the following reactions. The missing structure(s) can be a starting material or the major reaction product(s). H-Br CH2Cl2arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole



